Multi-invasions Are Recombination Byproducts that Induce Chromosomal Rearrangements
- PMID: 28781165
- PMCID: PMC5554464
- DOI: 10.1016/j.cell.2017.06.052
Multi-invasions Are Recombination Byproducts that Induce Chromosomal Rearrangements
Abstract
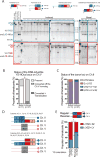
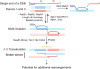
Inaccurate repair of broken chromosomes generates structural variants that can fuel evolution and inflict pathology. We describe a novel rearrangement mechanism in which translocation between intact chromosomes is induced by a lesion on a third chromosome. This multi-invasion-induced rearrangement (MIR) stems from a homologous recombination byproduct, where a broken DNA end simultaneously invades two intact donors. No homology is required between the donors, and the intervening sequence from the invading molecule is inserted at the translocation site. MIR is stimulated by increasing homology length and spatial proximity of the donors and depends on the overlapping activities of the structure-selective endonucleases Mus81-Mms4, Slx1-Slx4, and Yen1. Conversely, the 3'-flap nuclease Rad1-Rad10 and enzymes known to disrupt recombination intermediates (Sgs1-Top3-Rmi1, Srs2, and Mph1) inhibit MIR. Resolution of MIR intermediates propagates secondary chromosome breaks that frequently cause additional rearrangements. MIR features have implications for the formation of simple and complex rearrangements underlying human pathologies.
Keywords: D-loop; chromothripsis; endonuclease; genomic instability; helicase; homologous recombination; homology search; multi-invasion; translocation.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
AP and WDH conceived the project and wrote the manuscript, with input and editing from WW. AP designed, performed and analyzed the
Figures





References
-
- Binz SK, Dickson AM, Haring SJ, Wold MS. Functional assays for replication protein A (RPA) Methods Enzymol. 2006;409:11–38. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

