Clinical Characteristics of Seven Patients with Lanthanum Phosphate Deposition in the Stomach
- PMID: 28781325
- PMCID: PMC5596266
- DOI: 10.2169/internalmedicine.8720-16
Clinical Characteristics of Seven Patients with Lanthanum Phosphate Deposition in the Stomach
Abstract
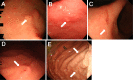
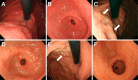
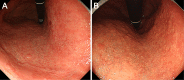
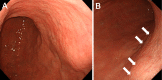
Objective To analyze the clinical characteristics and endoscopic features of patients with lanthanum deposition in the stomach. Patients We retrospectively reviewed seven patients with lanthanum deposition in the stomach who were diagnosed at Okayama Saiseikai General Hospital. We investigated the patient sex, age at diagnosis, medical and medication histories, gastrointestinal symptoms, complications, presence or absence of gastric atrophy, and outcome. We also investigated any changes in the endoscopic features if previous endoscopic images were available. Results Seven patients (six males and one female) had lanthanum deposition. The median age was 65 years (range, 50-79 years). All patients had been undergoing dialysis (continuous ambulatory peritoneal dialysis in one patient, hemodialysis in six patients). The dialysis period ranged from 16 to 73 months (median, 52 months). The patients had all been taking lanthanum carbonate for a period ranging from 5 to 45 months (median, 27 months). Gastric atrophy was noted in 6 patients (85.7%). One patient had difficulty swallowing, and 1 other patient had appetite loss. The other 5 patients were asymptomatic. Endoscopic features included annular whitish mucosa (n = 4), diffuse whitish mucosa (n = 3), and whitish spots (n = 2). Five patients underwent multiple esophagogastroduodenoscopy. The endoscopic features were unchanged in 2 patients, whereas the whitish mucosa became apparent and spread during the course in 3 patients. Conclusion We identified 7 patients with lanthanum deposition in the stomach. All patients showed whitish lesions macroscopically. Although the pathogenicity of gastric lanthanum deposition is uncertain, lanthanum-related lesions in the stomach progressed during continuous lanthanum phosphate intake in several patients.
Keywords: chronic kidney disease; hyperphosphatemia; lanthanum carbonate.
Figures

References
-
- Delmez JA, Slatopolsky E. Hyperphosphatemia: its consequences and treatment in patients with chronic renal disease. Am J Kidney Dis 19: 303-317, 1992. - PubMed
-
- Shigematsu T; Lanthanum Carbonate Research Group.. One year efficacy and safety of lanthanum carbonate for hyperphosphatemia in Japanese chronic kidney disease patients undergoing hemodialysis. Ther Apher Dial 14: 12-19, 2010. - PubMed
-
- Tsuchida K, Nagai K, Yokota N, Minakuchi J, Kawashima S. Impact of lanthanum carbonate on prognosis of chronic hemodialysis patients: a retrospective cohort study (Kawashima Study). Ther Apher Dial 20: 142-148, 2016. - PubMed
-
- Giotta N, Marino AM. Pharmacoeconomic analysis: analysis of cost-effectiveness of lanthanum-carbonate (Lc) in uncontrolled hyperphosphatemia in dialysis. Value Health 18: A511, 2015.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

