Fluorene-containing tetraphenylethylene molecules as lasing materials
- PMID: 28781425
- PMCID: PMC5516184
- DOI: 10.1002/pola.28421
Fluorene-containing tetraphenylethylene molecules as lasing materials
Abstract
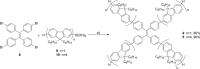
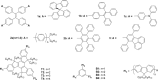
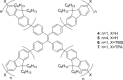
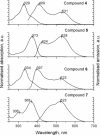
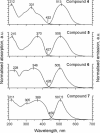
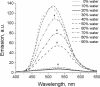
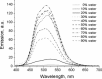
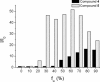
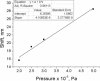
A series of star-shaped oligofluorene molecules, each containing a TPE core, have been specifically designed and produced to show effective aggregation-induced emission (AIE). Each molecule differs either in the number of fluorene units within the arms (e.g., 1 or 4, compounds 4 and 5), or the terminal group positioned at the end of each arm (e.g., H, TMS, or TPA, compounds 4, 6, and 7). Although they are all poor emitters in solution phase they become efficient yellow-green luminogens in the condensed state. Their AIE properties were investigated in THF/H2O mixtures, with each molecule exhibiting a clear emission enhancement at specific water contents. An all-organic distributed feedback (DFB) laser was fabricated using compound 4 as the gain material and exhibited an average threshold energy fluence of 60 ± 6 μJ/cm2 and emission in the green region. Furthermore, piezofluorochromism studies on a thin film of this material displayed a linear dependence of the amplified spontaneous emission (ASE) peak position on applied pressure, indicating potential applications as lasing-based pressure sensors. © 2016 The Authors. Journal of Polymer Science Part A: Polymer Chemistry Published by Wiley Periodicals, Inc. J. Polym. Sci., Part A: Polym. Chem. 2017, 55, 734-746.
Keywords: Synthesis; aggregation induced emission; oligomers; photophysics; piezofluorochromism.
Figures




References
-
- Valeur B., Molecular Fluorescence: Principles and Applications; Weinheim, FRG: Wiley‐VCH Verlag GmbH, 2001, pp. 72–124.
-
- Skabara P. J., Arlin J. B., Geerts Y. H., Adv. Mater. 2013, 25, 1948–1954. - PubMed
-
- Kanibolotsky A. L., Perepichka I. F., Skabara P. J., Chem. Soc. Rev. 2010, 39, 2695–2728. - PubMed
-
- Vithanage D. A., Manousiadis P. P., Sajjad M. T., Rajbhandari S., Chun H., Orofino C., Cortizo‐Lacalle D., Kanibolotsky A. L., Faulkner G., Findlay N. J., O'Brien D. C., Skabara P. J., Samuel I. D. W., Turnbull G. A., Appl. Phys. Lett. 2016, 109, 013302.
-
- Sajjad M. T., Manousiadis P. P., Orofino C., Kanibolotsky A. L., Findlay N. J., Rajbhandari S., Vithanage D. A., Chun H., Faulkner G., O'Brien D. C., Skabara P. J., Turnbull G. A., Samuel I. D. W., Appl. Phys. Lett. submitted.
LinkOut - more resources
Full Text Sources
Other Literature Sources
