Ginsenoside Rg1 prevents starvation-induced muscle protein degradation via regulation of AKT/mTOR/FoxO signaling in C2C12 myotubes
- PMID: 28781621
- PMCID: PMC5526193
- DOI: 10.3892/etm.2017.4615
Ginsenoside Rg1 prevents starvation-induced muscle protein degradation via regulation of AKT/mTOR/FoxO signaling in C2C12 myotubes
Abstract
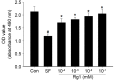
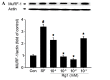
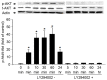
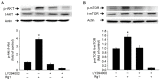
Skeletal muscle atrophy is often caused by catabolic conditions including fasting, disuse, aging and chronic diseases, such as chronic obstructive pulmonary disease. Atrophy occurs when the protein degradation rate exceeds the rate of protein synthesis. Therefore, maintaining a balance between the synthesis and degradation of protein in muscle cells is a major way to prevent skeletal muscle atrophy. Ginsenoside Rg1 (Rg1) is a primary active ingredient in Panax ginseng, which is considered to be one of the most valuable herbs in traditional Chinese medicine. In the current study, Rg1 was observed to inhibit the expression of MuRF-1 and atrogin-1 in C2C12 muscle cells in a starvation model. Rg1 also activated the phosphorylation of mammalian target of rapamycin (mTOR), protein kinase B (AKT), and forkhead transcription factor O, subtypes 1 and 3a. This phosphorylation was inhibited by LY294002, a phosphatidylinositol 3-kinase inhibitor. These data suggest that Rg1 may participate in the regulation of the balance between protein synthesis and degradation, and that the function of Rg1 is associated with the AKT/mTOR/FoxO signaling pathway.
Keywords: C2C12; ginsenoside Rg1; proteolysis; ubiquitin.
Figures


Similar articles
-
β-Hydroxy-β-methylbutyrate facilitates PI3K/Akt-dependent mammalian target of rapamycin and FoxO1/3a phosphorylations and alleviates tumor necrosis factor α/interferon γ-induced MuRF-1 expression in C2C12 cells.Nutr Res. 2014 Apr;34(4):368-74. doi: 10.1016/j.nutres.2014.02.003. Epub 2014 Feb 10. Nutr Res. 2014. PMID: 24774073
-
Ginsenoside Rg1 Attenuates Muscle Atrophy in Hyperglycemic Conditions, Inactivity and Protein Deprivation Models via AKT/mTOR Pathway Activation.Curr Mol Med. 2025 Apr 23. doi: 10.2174/0115665240355315250414051525. Online ahead of print. Curr Mol Med. 2025. PMID: 40277061
-
Prevention of skeletal muscle atrophy in vitro using anti-ubiquitination oligopeptide carried by atelocollagen.Biochim Biophys Acta. 2015 May;1853(5):873-80. doi: 10.1016/j.bbamcr.2015.01.024. Epub 2015 Feb 7. Biochim Biophys Acta. 2015. PMID: 25667084
-
PI3 kinase regulation of skeletal muscle hypertrophy and atrophy.Curr Top Microbiol Immunol. 2010;346:267-78. doi: 10.1007/82_2010_78. Curr Top Microbiol Immunol. 2010. PMID: 20593312 Review.
-
Glucocorticoid-induced skeletal muscle atrophy.Int J Biochem Cell Biol. 2013 Oct;45(10):2163-72. doi: 10.1016/j.biocel.2013.05.036. Epub 2013 Jun 24. Int J Biochem Cell Biol. 2013. PMID: 23806868 Review.
Cited by
-
Glucose, glutamine, lactic acid and α‑ketoglutarate restore metabolic disturbances and atrophic changes in energy‑deprived muscle cells.Mol Med Rep. 2025 Jul;32(1):197. doi: 10.3892/mmr.2025.13562. Epub 2025 May 16. Mol Med Rep. 2025. PMID: 40376969 Free PMC article.
-
Korean red ginseng suppresses mitochondrial apoptotic pathway in denervation-induced skeletal muscle atrophy.J Ginseng Res. 2024 Jan;48(1):52-58. doi: 10.1016/j.jgr.2023.07.002. Epub 2023 Jul 5. J Ginseng Res. 2024. PMID: 38223821 Free PMC article.
-
Transcriptome and Methylome Profiling in Rat Skeletal Muscle: Impact of Post-Weaning Protein Restriction.Int J Mol Sci. 2022 Dec 12;23(24):15771. doi: 10.3390/ijms232415771. Int J Mol Sci. 2022. PMID: 36555412 Free PMC article.
-
Ginsenoside Rg1 promotes browning by inducing UCP1 expression and mitochondrial activity in 3T3-L1 and subcutaneous white adipocytes.J Ginseng Res. 2019 Oct;43(4):589-599. doi: 10.1016/j.jgr.2018.07.005. Epub 2018 Jul 18. J Ginseng Res. 2019. PMID: 31695565 Free PMC article.
-
Ginsenoside Rc, an Active Component of Panax ginseng, Alleviates Oxidative Stress-Induced Muscle Atrophy via Improvement of Mitochondrial Biogenesis.Antioxidants (Basel). 2023 Aug 7;12(8):1576. doi: 10.3390/antiox12081576. Antioxidants (Basel). 2023. PMID: 37627571 Free PMC article.
References
-
- Kawai N, Hirasaka K, Maeda T, Haruna M, Shiota C, Ochi A, Abe T, Kohno S, Ohno A, Teshima-Kondo S, et al. Prevention of skeletal muscle atrophy in vitro using anti-ubiquitination oligopeptide carried by atelocollagen. Biochim Biophy Acta. 2015;1853:873–880. doi: 10.1016/j.bbamcr.2015.01.024. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
