A modular yeast biosensor for low-cost point-of-care pathogen detection
- PMID: 28782007
- PMCID: PMC5489263
- DOI: 10.1126/sciadv.1603221
A modular yeast biosensor for low-cost point-of-care pathogen detection
Abstract
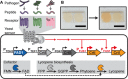
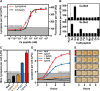
The availability of simple, specific, and inexpensive on-site detection methods is of key importance for deployment of pathogen surveillance networks. We developed a nontechnical and highly specific colorimetric assay for detection of pathogen-derived peptides based on Saccharomyces cerevisiae-a genetically tractable model organism and household product. Integrating G protein-coupled receptors with a visible, reagent-free lycopene readout, we demonstrate differential detection of major human, plant, and food fungal pathogens with nanomolar sensitivity. We further optimized a one-step rapid dipstick prototype that can be used in complex samples, including blood, urine, and soil. This modular biosensor can be economically produced at large scale, is not reliant on cold-chain storage, can be detected without additional equipment, and is thus a compelling platform scalable to global surveillance of pathogens.
Keywords: G-Protein coupled receptor; Yeast; biosensor; fungal pathogens; invasive fungal infections; low-cost diagnostic; lycopene; peptide biomarker; point-of-care; synthetic biology.
Figures


Similar articles
-
Cascaded amplifying circuit enables sensitive detection of fungal pathogens.Biosens Bioelectron. 2024 Apr 15;250:116058. doi: 10.1016/j.bios.2024.116058. Epub 2024 Jan 20. Biosens Bioelectron. 2024. PMID: 38281368
-
Evaluation and comparison of colorimetric outputs for yeast-based biosensors in laboratory and point-of-use settings.FEMS Microbiol Lett. 2024 Jan 9;371:fnae034. doi: 10.1093/femsle/fnae034. FEMS Microbiol Lett. 2024. PMID: 38782713 Free PMC article.
-
Engineering a GPCR-based yeast biosensor for a highly sensitive melatonin detection from fermented beverages.Sci Rep. 2024 Aug 1;14(1):17852. doi: 10.1038/s41598-024-68633-y. Sci Rep. 2024. PMID: 39090231 Free PMC article.
-
Difficulties with molecular diagnostic tests for mould and yeast infections: where do we stand?Clin Microbiol Infect. 2014 Jun;20 Suppl 6:36-41. doi: 10.1111/1469-0691.12617. Clin Microbiol Infect. 2014. PMID: 24661790 Review.
-
Innovative technologies for point-of-care testing of viral hepatitis in low-resource and decentralized settings.J Viral Hepat. 2018 Feb;25(2):108-117. doi: 10.1111/jvh.12827. Epub 2017 Dec 27. J Viral Hepat. 2018. PMID: 29134742 Review.
Cited by
-
Engineering a Model Cell for Rational Tuning of GPCR Signaling.Cell. 2019 Apr 18;177(3):782-796.e27. doi: 10.1016/j.cell.2019.02.023. Epub 2019 Apr 4. Cell. 2019. PMID: 30955892 Free PMC article.
-
Paper-Based Humidity Sensors as Promising Flexible Devices: State of the Art: Part 1. General Consideration.Nanomaterials (Basel). 2023 Mar 20;13(6):1110. doi: 10.3390/nano13061110. Nanomaterials (Basel). 2023. PMID: 36986004 Free PMC article. Review.
-
Improving engineered biological systems with electronics and microfluidics.Nat Biotechnol. 2025 Jul;43(7):1067-1083. doi: 10.1038/s41587-025-02709-6. Epub 2025 Jun 27. Nat Biotechnol. 2025. PMID: 40579601 Review.
-
Porous Cellulose Substrate Study to Improve the Performance of Diffusion-Based Ionic Strength Sensors.Membranes (Basel). 2022 Oct 29;12(11):1074. doi: 10.3390/membranes12111074. Membranes (Basel). 2022. PMID: 36363629 Free PMC article.
-
Synthetic biology-inspired cell engineering in diagnosis, treatment, and drug development.Signal Transduct Target Ther. 2023 Mar 11;8(1):112. doi: 10.1038/s41392-023-01375-x. Signal Transduct Target Ther. 2023. PMID: 36906608 Free PMC article. Review.
References
-
- Institute of Medicine, Forum on Microbial Threats, Global Infectious Disease Surveillance and Detection: Assessing the Challenges—Finding Solutions, Workshop Summary (National Academies Press, 2007). - PubMed
-
- Bissonnette L., Bergeron M. G., Diagnosing infections—Current and anticipated technologies for point-of-care diagnostics and home-based testing. Clin. Microbiol. Infect. 16, 1044–1053 (2010). - PubMed
-
- Singh R., Mukherjee M. D., Sumana G., Gupta R. K., Sood S., Malhotra B. D., Biosensors for pathogen detection: A smart approach towards clinical diagnosis. Sens. Actuators B Chem. 197, 385–404 (2014).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

