Going Deeper: Biomolecular Tools for Acoustic and Magnetic Imaging and Control of Cellular Function
- PMID: 28782927
- PMCID: PMC6058970
- DOI: 10.1021/acs.biochem.7b00443
Going Deeper: Biomolecular Tools for Acoustic and Magnetic Imaging and Control of Cellular Function
Abstract
Most cellular phenomena of interest to mammalian biology occur within the context of living tissues and organisms. However, today's most advanced tools for observing and manipulating cellular function, based on fluorescent or light-controlled proteins, work best in cultured cells, transparent model species, or small, surgically accessed anatomical regions. Their reach into deep tissues and larger animals is limited by photon scattering. To overcome this limitation, we must design biochemical tools that interface with more penetrant forms of energy. For example, sound waves and magnetic fields easily permeate most biological tissues, allowing the formation of images and delivery of energy for actuation. These capabilities are widely used in clinical techniques such as diagnostic ultrasound, magnetic resonance imaging, focused ultrasound ablation, and magnetic particle hyperthermia. Each of these modalities offers spatial and temporal precision that could be used to study a multitude of cellular processes in vivo. However, connecting these techniques to cellular functions such as gene expression, proliferation, migration, and signaling requires the development of new biochemical tools that can interact with sound waves and magnetic fields as optogenetic tools interact with photons. Here, we discuss the exciting challenges this poses for biomolecular engineering and provide examples of recent advances pointing the way to greater depth in in vivo cell biology.
Conflict of interest statement
COMPETING INTERESTS
The authors declare no competing financial interests.
Figures


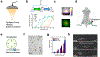

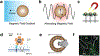
References
-
- Foster FS, Pavlin CJ, Harasiewicz KA, Christopher DA, and Turnbull DH (2000) Advances in ultrasound biomicroscopy. Ultrasound Med. Biol 26, 1–27. - PubMed
-
- Errico C, Pierre J, Pezet S, Desailly Y, Lenkei Z, Couture O, and Tanter M (2015) Ultrafast ultrasound localization microscopy for deep super-resolution vascular imaging. Nature 527, 499–502. - PubMed
-
- Humphrey VF (2007) Ultrasound and matter-Physical interactions. Prog. Biophys. Mol. Biol 93, 195–211. - PubMed
-
- Pankhurst QA, Connolly J, Jones SK, and Dobson J (2003) Applications of magnetic nanoparticles in biomedicine. J. Phys. D. Appl. Phys 36, R167–R181.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

