Regulation of Focal Adhesion Kinase through a Direct Interaction with an Endogenous Inhibitor
- PMID: 28782937
- PMCID: PMC6800154
- DOI: 10.1021/acs.biochem.7b00616
Regulation of Focal Adhesion Kinase through a Direct Interaction with an Endogenous Inhibitor
Abstract
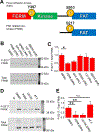
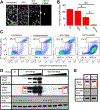
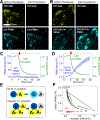
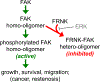
Focal adhesion kinase (FAK) plays a key role in integrin and growth factor signaling pathways. FAK-related non-kinase (FRNK) is an endogenous inhibitor of FAK that shares its primary structure with the C-terminal third of FAK. FAK S910 phosphorylation is known to regulate FAK protein-protein interactions, but the role of the equivalent site on FRNK (S217) is unknown. Here we determined that S217 is highly phosphorylated by ERK in cultured rat aortic smooth muscle cells. Blocking phosphorylation by mutation (S217A) greatly increased FRNK inhibitory potency, resulting in strong inhibition of FAK autophosphorylation at Y397 and induction of smooth muscle cell apoptosis. FRNK has been proposed to compete for FAK anchoring sites in focal adhesions, but we did not detect displacement of FAK by WT-FRNK or superinhibitory S217A-FRNK. Instead, we found FRNK interacted directly with FAK, and this interaction is markedly strengthened for the superinhibitory S217A-FRNK. The results suggest that FRNK limits growth and survival signaling pathways by binding directly to FAK in an inhibitory complex, and this inhibition is relieved by phosphorylation of FRNK at S217.
Conflict of interest statement
Conflict of interest: The authors declare that they have no conflicts of interest with the contents of this article.
Figures


References
-
- Govindarajan G, Eble DM, Lucchesi PA, and Samarel AM (2000) Focal adhesion kinase is involved in angiotensin II-mediated protein synthesis in cultured vascular smooth muscle cells, Circ. Res 87, 710–716. - PubMed
-
- Heidkamp MC, Bayer AL, Kalina JA, Eble DM, and Samarel AM (2002) GFP-FRNK disrupts focal adhesions and induces anoikis in neonatal rat ventricular myocytes, Circ. Res 90, 1282–1289. - PubMed
-
- Walkiewicz KW, Girault JA, and Arold ST (2015) How to awaken your nanomachines: Site-specific activation of focal adhesion kinases through ligand interactions, Prog. Biophys. Mol. Biol 119, 60–71. - PubMed
-
- Parsons JT (2003) Focal adhesion kinase: the first ten years, J. Cell Sci 116, 1409–1416. - PubMed
-
- Mitra SK, Hanson DA, and Schlaepfer DD (2005) Focal adhesion kinase: in command and control of cell motility, Nat. Rev. Mol. Cell Biol 6, 56–68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

