ErbB2-positive mammary tumors can escape PI3K-p110α loss through downregulation of the Pten tumor suppressor
- PMID: 28783168
- PMCID: PMC5808977
- DOI: 10.1038/onc.2017.264
ErbB2-positive mammary tumors can escape PI3K-p110α loss through downregulation of the Pten tumor suppressor
Abstract
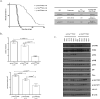
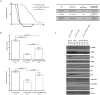
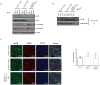
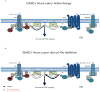
Breast cancer is the most common cancer among women and 30% of patients will be diagnosed with an ErbB2-positive tumor. Forty percent of ErbB2-positive breast tumors have an activating mutation in p110α, a catalytic subunit of phosphoinositide 3-kinase. Clinical and experimental data show that breast tumors treated with a p110α-specific inhibitor often circumvent inhibition and resume growth. To understand this mechanism of resistance, we crossed a p110α conditional (p110αflx/flx) mouse model with mice that overexpress the ErbB2/Neu-IRES-Cre transgene (NIC) specifically in the mammary epithelium. Although mammary-specific deletion of p110α dramatically delays tumor onset, tumors eventually arise and are dependent on p110β. Through biochemical analyses we find that a proportion of p110α-deficient tumors (23%) display downregulation of the Pten tumor suppressor. We further demonstrate that loss of one allele of PTEN is sufficient to shift isoform dependency from p110α to p110β in vivo. These results provide insight into the molecular mechanism by which ErbB2-positive breast cancer escapes p110α inhibition.
Conflict of interest statement
Figures

References
-
- Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. 2006;7(8):606–619. http://doi.org/10.1038/nrg1879. - DOI - PubMed
-
- Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. 2010;11(5):329–341. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

