Fibroblast growth factor receptor is a mechanistic link between visceral adiposity and cancer
- PMID: 28783178
- PMCID: PMC5709202
- DOI: 10.1038/onc.2017.278
Fibroblast growth factor receptor is a mechanistic link between visceral adiposity and cancer
Abstract
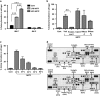
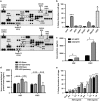
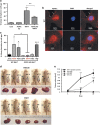
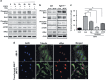
Epidemiological evidence implicates excess adipose tissue in increasing cancer risk. Despite a steeply rising global prevalence of obesity, how adiposity contributes to transformation (stage a non-tumorigenic cell undergoes to become malignant) is unknown. To determine the factors in adipose tissue that stimulate transformation, we used a novel ex vivo system of visceral adipose tissue (VAT)-condition medium-stimulated epithelial cell growth in soft agar. To extend this system in vivo, we used a murine lipectomy model of ultraviolet light B-induced, VAT-promoted skin tumor formation. We found that VAT from mice and obese human donors stimulated growth in soft agar of non-tumorigenic epithelial cells. The difference in VAT activity was associated with fibroblast growth factor-2 (FGF2) levels. Moreover, human and mouse VAT failed to stimulate growth in soft of agar in cells deficient in FGFR-1 (FGF2 receptor). We also demonstrated that circulating levels of FGF2 were associated with non-melanoma tumor formation in vivo. These data implicate FGF2 as a major factor VAT releases to transform epithelial cells-a novel, potential pathway of VAT-enhanced tumorigenesis. Strategies designed to deplete VAT stores of FGF2 or inhibit FGFR-1 in abdominally obese individuals may be important cancer prevention strategies as well as adjuvant therapies for improving outcomes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
A BET Bromodomain Inhibitor Suppresses Adiposity-Associated Malignant Transformation.Cancer Prev Res (Phila). 2018 Mar;11(3):129-142. doi: 10.1158/1940-6207.CAPR-17-0262. Epub 2017 Dec 15. Cancer Prev Res (Phila). 2018. PMID: 29246955
-
A role for FGF2 in visceral adiposity-associated mammary epithelial transformation.Adipocyte. 2018;7(2):113-120. doi: 10.1080/21623945.2018.1445889. Epub 2018 Mar 21. Adipocyte. 2018. PMID: 29561195 Free PMC article.
-
The Use of Human Serum Samples to Study Malignant Transformation: A Pilot Study.Cells. 2021 Oct 6;10(10):2670. doi: 10.3390/cells10102670. Cells. 2021. PMID: 34685650 Free PMC article.
-
Elucidating the role of adipose tissue secreted factors in malignant transformation.Adipocyte. 2018 Jan 2;7(1):45-48. doi: 10.1080/21623945.2017.1388971. Epub 2017 Nov 2. Adipocyte. 2018. PMID: 29095087 Free PMC article.
-
Visceral adiposity and inflammatory bowel disease.Int J Colorectal Dis. 2021 Nov;36(11):2305-2319. doi: 10.1007/s00384-021-03968-w. Epub 2021 Jun 9. Int J Colorectal Dis. 2021. PMID: 34104989 Review.
Cited by
-
Implications of obesity and insulin resistance for the treatment of oestrogen receptor-positive breast cancer.Br J Cancer. 2024 Dec;131(11):1724-1736. doi: 10.1038/s41416-024-02833-1. Epub 2024 Sep 9. Br J Cancer. 2024. PMID: 39251829 Free PMC article. Review.
-
Nonalcoholic fatty liver disease and colorectal cancer: Correlation and missing links.Life Sci. 2020 Dec 1;262:118507. doi: 10.1016/j.lfs.2020.118507. Epub 2020 Oct 2. Life Sci. 2020. PMID: 33017572 Free PMC article. Review.
-
A risk-associated Active transcriptome phenotype expressed by histologically normal human breast tissue and linked to a pro-tumorigenic adipocyte population.Breast Cancer Res. 2020 Jul 31;22(1):81. doi: 10.1186/s13058-020-01322-6. Breast Cancer Res. 2020. PMID: 32736587 Free PMC article.
-
The influence of fibroblast growth factor 2 on the senescence of human adipose-derived mesenchymal stem cells during long-term culture.Stem Cells Transl Med. 2020 Apr;9(4):518-530. doi: 10.1002/sctm.19-0234. Epub 2019 Dec 16. Stem Cells Transl Med. 2020. PMID: 31840944 Free PMC article.
-
Adipose tissue-secreted Spz5 promotes distal tumor progression via Toll-6-mediated Hh pathway activation in Drosophila.EMBO J. 2025 Aug;44(15):4301-4330. doi: 10.1038/s44318-025-00489-y. Epub 2025 Jun 23. EMBO J. 2025. PMID: 40551010 Free PMC article.
References
-
- Renehan AG, Zwahlen M, Egger M. Adiposity and cancer risk: new mechanistic insights from epidemiology. Nat Rev Cancer 2015; 15: 484–498. - PubMed
-
- Nunez NP, Perkins SN, Smith NC, Berrigan D, Berendes DM, Varticovski L et al. Obesity accelerates mouse mammary tumor growth in the absence of ovarian hormones. Nutr Cancer 2008; 60: 534–541. - PubMed
-
- Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA 2010; 303: 242–249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

