Off-the-Shelf Virus-Specific T Cells to Treat BK Virus, Human Herpesvirus 6, Cytomegalovirus, Epstein-Barr Virus, and Adenovirus Infections After Allogeneic Hematopoietic Stem-Cell Transplantation
- PMID: 28783452
- PMCID: PMC5662844
- DOI: 10.1200/JCO.2017.73.0655
Off-the-Shelf Virus-Specific T Cells to Treat BK Virus, Human Herpesvirus 6, Cytomegalovirus, Epstein-Barr Virus, and Adenovirus Infections After Allogeneic Hematopoietic Stem-Cell Transplantation
Abstract
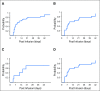
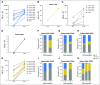
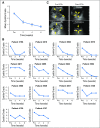
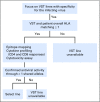
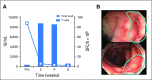
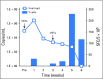
Purpose Improvement of cure rates for patients treated with allogeneic hematopoietic stem-cell transplantation (HSCT) will require efforts to decrease treatment-related mortality from severe viral infections. Adoptively transferred virus-specific T cells (VSTs) generated from eligible, third-party donors could provide broad antiviral protection to recipients of HSCT as an immediately available off-the-shelf product. Patient and Methods We generated a bank of VSTs that recognized five common viral pathogens: Epstein-Barr virus (EBV), adenovirus (AdV), cytomegalovirus (CMV), BK virus (BKV), and human herpesvirus 6 (HHV-6). The VSTs were administered to 38 patients with 45 infections in a phase II clinical trial. Results A single infusion produced a cumulative complete or partial response rate of 92% (95% CI, 78.1% to 98.3%) overall and the following rates by virus: 100% for BKV (n = 16), 94% for CMV (n = 17), 71% for AdV (n = 7), 100% for EBV (n = 2), and 67% for HHV-6 (n = 3). Clinical benefit was achieved in 31 patients treated for one infection and in seven patients treated for multiple coincident infections. Thirteen of 14 patients treated for BKV-associated hemorrhagic cystitis experienced complete resolution of gross hematuria by week 6. Infusions were safe, and only two occurrences of de novo graft-versus host disease (grade 1) were observed. VST tracking by epitope profiling revealed persistence of functional VSTs of third-party origin for up to 12 weeks. Conclusion The use of banked VSTs is a feasible, safe, and effective approach to treat severe and drug-refractory infections after HSCT, including infections from two viruses (BKV and HHV-6) that had never been targeted previously with an off-the-shelf product. Furthermore, the multispecificity of the VSTs ensures extensive antiviral coverage, which facilitates the treatment of patients with multiple infections.
Figures

References
-
- Gratwohl A, Brand R, Frassoni F, et al. : Cause of death after allogeneic hematopoietic stem cell transplantation (HSCT) in early leukaemias: An EBMT analysis of lethal infectious complications and changes over calendar time. Bone Marrow Transplant 36:757-769, 2005 - PubMed
-
- Styczynski J, Einsele H, Gil L, et al. : Outcome of treatment of Epstein-Barr virus-related post-transplant lymphoproliferative disorder in hematopoietic stem cell recipients: A comprehensive review of reported cases. Transpl Infect Dis 11:383-392, 2009 - PubMed
-
- Gilis L, Morisset S, Billaud G, et al. : High burden of BK virus-associated hemorrhagic cystitis in patients undergoing allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 49:664-670, 2014 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

