Immune perturbations in HIV-1-infected individuals who make broadly neutralizing antibodies
- PMID: 28783677
- PMCID: PMC5589960
- DOI: 10.1126/sciimmunol.aag0851
Immune perturbations in HIV-1-infected individuals who make broadly neutralizing antibodies
Abstract
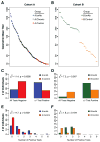
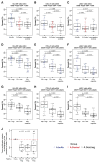
Induction of broadly neutralizing antibodies (bnAbs) is a goal of HIV-1 vaccine development. bnAbs occur in some HIV-1-infected individuals and frequently have characteristics of autoantibodies. We have studied cohorts of HIV-1-infected individuals who made bnAbs and compared them with those who did not do so, and determined immune traits associated with the ability to produce bnAbs. HIV-1-infected individuals with bnAbs had a higher frequency of blood autoantibodies, a lower frequency of regulatory CD4+ T cells, a higher frequency of circulating memory T follicular helper CD4+ cells, and a higher T regulatory cell level of programmed cell death-1 expression compared with HIV-1-infected individuals without bnAbs. Thus, induction of HIV-1 bnAbs may require vaccination regimens that transiently mimic immunologic perturbations in HIV-1-infected individuals.
Copyright © 2016, American Association for the Advancement of Science.
Figures



Comment in
-
HIV: A recipe for inducing broadly neutralizing antibodies.Nat Rev Immunol. 2016 Sep;16(9):533. doi: 10.1038/nri.2016.92. Epub 2016 Aug 8. Nat Rev Immunol. 2016. PMID: 27498765 No abstract available.
References
-
- Fauci AS, Marston HD. Ending AIDS--is an HIV vaccine necessary? N Engl J Med. 2014;370:495–498. - PubMed
-
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH. Vaccination with ALVAC and AIDSVAX to Prevent HIV-1 Infection in Thailand. N Engl J Med. 2009;361:2209–2220. - PubMed
-
- Montefiori DC, Karnasuta C, Huang Y, Ahmed H, Gilbert P, de Souza MS, mclinden R, Tovanabutra S, Laurence-Chenine A, Sanders-Buell E, Moody MA, Bonsignori M, Ochsenbauer C, Kappes J, Tang H, Greene K, Gao H, Labranche CC, Andrews C, Polonis VR, Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Self SG, Berman PW, Francis D, Sinangil F, Lee C, Tartaglia J, Robb ML, Haynes BF, Michael NL, Kim JH. Magnitude and breadth of the neutralizing antibody response in the RV144 and Vax003 HIV-1 vaccine efficacy trials. Journal of Infectious Diseases. 2012;206:431–441. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

