Motivations, enrollment decisions, and socio-demographic characteristics of healthy volunteers in phase 1 research
- PMID: 28783972
- PMCID: PMC9896431
- DOI: 10.1177/1740774517722130
Motivations, enrollment decisions, and socio-demographic characteristics of healthy volunteers in phase 1 research
Abstract
Background/aim: Phase 1 trials with healthy volunteers are an integral step in drug development. Commentators worry about the possible exploitation of healthy volunteers because they are assumed to be disadvantaged, marginalized, and inappropriately influenced by the offer of money for research for which they do not appreciate the inherent risks. Yet there are limited data to support or refute these concerns. This study aims to describe the socio-demographic characteristics, motivations, and enrollment decision-making of a large cohort of healthy volunteers.
Methods: We used a cross-sectional anonymous survey of 1194 healthy volunteers considering enrollment in phase 1 studies at Pfizer Clinical Research Units in New Haven, CT; Brussels, Belgium; and Singapore. Descriptive statistics describe motivations and socio-demographic characteristics. Comparisons between groups were examined.
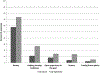
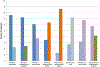
Results: The majority rated consideration of risks as more important to their enrollment decision than the amount of money, despite reporting that their primary motivation was financial. Risk, time, money, the competence and friendliness of research staff, and contributing to medical research were important factors influencing enrollment decisions for most participants. The majority of healthy volunteers in this cohort were male, single, reported higher than high school education, and 70% had previous research experience. Many reported low annual incomes (50% below USD$25,000) and high rates of unemployment (33% overall). Nonetheless, risk as an important consideration, money, and other reported considerations and motivations, except for time, did not vary by income, employment, education, or previous experience. There were regional differences in both socio-demographic characteristics and factors important to participation decisions.
Conclusion: Healthy volunteers in phase 1 studies consider risks as more important to their enrollment decisions than the amount of money offered, although most are motivated to participate by the offer of money. Healthy volunteers are indeed low income, disproportionately unemployed, and have significant prior research experience. Yet these factors do not appear to affect either their motivations for participation or factors important to their research enrollment decisions.
Keywords: Healthy volunteers; financial incentives; motivations.
Conflict of interest statement
Declaration of conflicting interests
Dr. Emanuel is a frequent paid event speaker at conventions, committee meetings and professional healthcare gatherings represented by the Leigh Bureau. He is also venture partner with Oak HC/FT and a Nuna stockholder. The other authors declared that they have no conflicting interests to report.
Figures
Comment in
-
Response.Clin Trials. 2017 Oct;14(5):551-552. doi: 10.1177/1740774517722127. Epub 2017 Jul 14. Clin Trials. 2017. PMID: 28709387 Free PMC article. No abstract available.
-
Commentary on Grady et al.: Using poor, uninsured minorities to test the safety of experimental drugs.Clin Trials. 2017 Oct;14(5):547-550. doi: 10.1177/1740774517722126. Epub 2017 Jul 27. Clin Trials. 2017. PMID: 28747074 No abstract available.
References
-
- ClinicalTrials.gov. Glossary of common site terms, https://www.clinicaltrials.gov/ct2/info/glossary#P (Accessed 10 August 2015)
-
- CenterWatch. Overview of clinical trials, http://www.centerwatch.com/clinicaltrials/overview.aspx (Accessed 15, November 2016).
-
- Pasqualetti G, Gori G, Blandizzi C, et al. Healthy volunteers and early phases of clinical experimentation. Eur J Clin Pharmacol 2010; 66: 647–653. - PubMed
-
- Agrawal M, Grady C, Fairclough DL, et al. Patients’ decision - making process regarding participation in phase I oncology research. J Clin Oncol 2006; 24: 4479–4484. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

