Controversial Ebola vaccine trials in Ghana: a thematic analysis of critiques and rebuttals in digital news
- PMID: 28784109
- PMCID: PMC5547580
- DOI: 10.1186/s12889-017-4618-8
Controversial Ebola vaccine trials in Ghana: a thematic analysis of critiques and rebuttals in digital news
Abstract
Background: Communication is of paramount importance in responding to health crises. We studied the media messages put forth by different stakeholders in two Ebola vaccine trials that became controversial in Ghana. These interactions between health authorities, political actors, and public citizens can offer key lessons for future research. Through an analysis of online media, we analyse stakeholder concerns and incentives, and the phases of the dispute, to understand how the dispute evolved to the point of the trials being suspended, and analyse what steps might have been taken to avert this outcome.
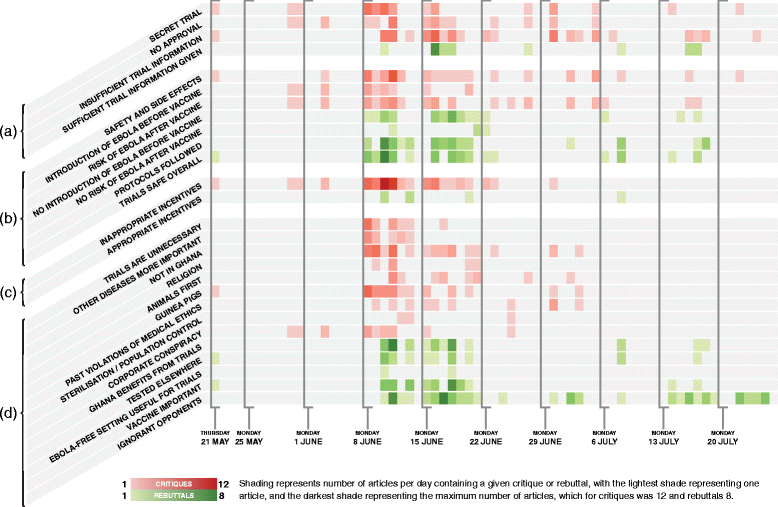
Methods: A web-based system was developed to download and analyse news reports relevant to Ebola vaccine trials. This included monitoring major online newspapers in each country with planned clinical trials, including Ghana. All news articles were downloaded, selecting out those containing variants of the words "Ebola," and "vaccine," which were analysed thematically by a team of three coders. Two types of themes were defined: critiques of the trials and rebuttals in favour of the trials. After reconciling differences between coders' results, the data were visualised and reviewed to describe and interpret the debate.
Results: A total of 27,460 articles, published between 1 May and 30 July 2015, were collected from nine different newspapers in Ghana, of which 139 articles contained the keywords and met the inclusion criteria. The final codebook included 27 themes, comprising 16 critiques and 11 rebuttals. After coding and reconciliation, the main critiques (and their associated rebuttals) were selected for in-depth analysis, including statements about the trials being secret (mentioned in 21% of articles), claims that the vaccine trials would cause an Ebola outbreak in Ghana (33%), and the alleged impropriety of the incentives offered to participants (35%).
Discussion: Perceptions that the trials were "secret" arose from a combination of premature news reporting and the fact that the trials were prohibited from conducting any publicity before being approved at the time that the story came out, which created an impression of secrecy. Fears about Ebola being spread in Ghana appeared in two forms, the first alleging that scientists would intentionally infect Ghanaians with Ebola in order to test the vaccine, and the second suggesting that the vaccine might give trial participants Ebola as a side-effect - over the course of the debate, the latter became the more prominent of the two variants. The incentives were sometimes criticised for being coercively large, but were much more often criticised for being too small, which may have been related to a misperception that the incentives were meant as compensation for the trials' risks, which were themselves exaggerated.
Conclusion: The rumours captured through this research indicate the variety of strong emotions drawn out by the trials, highlighting the importance of understanding the emotional and social context of such research. The uncertainty, fear, and distrust associated with the trials draw from the contemporary context of the Ebola outbreak, as well as longstanding historical issues in Ghana. By analysing the debate from its inception, we can see how the controversy unfolded, and identify points of concern that can inform health communication, suggesting that this tool may be valuable in future epidemics and crises.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
HL has served on the Merck Vaccine Strategic Advisory Board, and is the director of the Vaccine Confidence Project (VCP), which has received funds from Merck and GSK to convene research symposia and has advised GSK on vaccine hesitancy issues. WS also is a researcher with the VCP. The other authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures





References
-
- Chandler C, Fairhead J, Kelly A, Leach M, Martineau F, Mokuwa E, Parker M, Richards P, Wilkinson a; Ebola response anthropology platform. Ebola: limitations of correcting misinformation. Lancet 2015;385(9975):1275-1277. doi: 10.1016/S0140-6736(14)62382-5. Epub 2014 Dec 19. - PubMed
-
- Graphic Online. Parliament approves Ebola vaccine in the country. 2015. Available at: https://web.archive.org/web/20170728092805/http://www.graphic.com.gh/new.... Accessed 28 July 2017.
-
- Larson HJ, Brocard P, Garnett G. The India HPV-vaccine suspension. Lancet. 376 (2010):572–573. doi: 10.1016/S0140-6736(10)62094-6. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

