How Hsp90 and Cdc37 Lubricate Kinase Molecular Switches
- PMID: 28784328
- PMCID: PMC5621984
- DOI: 10.1016/j.tibs.2017.07.002
How Hsp90 and Cdc37 Lubricate Kinase Molecular Switches
Abstract
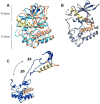
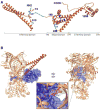
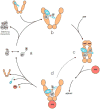
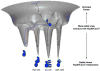
The Hsp90/Cdc37 chaperone system interacts with and supports 60% of the human kinome. Not only are Hsp90 and Cdc37 generally required for initial folding, but many kinases rely on the Hsp90/Cdc37 throughout their lifetimes. A large fraction of these 'client' kinases are key oncoproteins, and their interactions with the Hsp90/Cdc37 machinery are crucial for both their normal and malignant activity. Recently, advances in single-particle cryo-electron microscopy (cryoEM) and biochemical strategies have provided the first key molecular insights into kinase-chaperone interactions. The surprising results suggest a re-evaluation of the role of chaperones in the kinase lifecycle, and suggest that such interactions potentially allow kinases to more rapidly respond to key signals while simultaneously protecting unstable kinases from degradation and suppressing unwanted basal activity.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures

References
-
- Endicott JA, Noble ME, Johnson LN. The structural basis for control of eukaryotic protein kinases. Annu Rev Biochem. 2012;81:587–613. - PubMed
-
- Gierasch LM, Horwich A, Slingsby C, Wickner S, Agard D. Series in structural biology Vol 6. World Scientific; New Jersey: 2016. 1 online resource (vii, 319 pages)
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

