Adipose Stem Cell Therapy Mitigates Chronic Pancreatitis via Differentiation into Acinar-like Cells in Mice
- PMID: 28784560
- PMCID: PMC5675167
- DOI: 10.1016/j.ymthe.2017.06.016
Adipose Stem Cell Therapy Mitigates Chronic Pancreatitis via Differentiation into Acinar-like Cells in Mice
Abstract
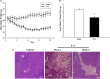
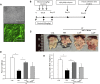
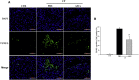
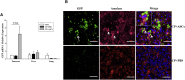
The objective of this study was to assess the capacity of adipose-derived mesenchymal stem cells (ASCs) to mitigate disease progression in an experimental chronic pancreatitis mouse model. Chronic pancreatitis (CP) was induced in C57BL/6 mice by repeated ethanol and cerulein injection, and mice were then infused with 4 × 105 or 1 × 106 GFP+ ASCs. Pancreas morphology, fibrosis, inflammation, and presence of GFP+ ASCs in pancreases were assessed 2 weeks after treatment. We found that ASC infusion attenuated pancreatic damage, preserved pancreas morphology, and reduced pancreatic fibrosis and cell death. GFP+ ASCs migrated to pancreas and differentiated into amylase+ cells. In further confirmation of the plasticity of ASCs, ASCs co-cultured with acinar cells in a Transwell system differentiated into amylase+ cells with increased expression of acinar cell-specific genes including amylase and chymoB1. Furthermore, culture of acinar or pancreatic stellate cell lines in ASC-conditioned medium attenuated ethanol and cerulein-induced pro-inflammatory cytokine production in vitro. Our data show that a single intravenous injection of ASCs ameliorated CP progression, likely by directly differentiating into acinar-like cells and by suppressing inflammation, fibrosis, and pancreatic tissue damage. These results suggest that ASC cell therapy has the potential to be a valuable treatment for patients with pancreatitis.
Keywords: acinar cells; adipose stem cell therapy; differentiation; ethanol and cerulean-induced chronic pancreatitis mouse model; fibrosis; inflammation; mesenchymal stem cells.
Copyright © 2017 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Figures

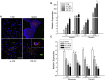
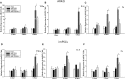
Comment in
-
Adipose Stem Cell Therapy for Chronic Pancreatitis.Mol Ther. 2017 Nov 1;25(11):2438-2439. doi: 10.1016/j.ymthe.2017.10.007. Epub 2017 Oct 19. Mol Ther. 2017. PMID: 29055621 Free PMC article. No abstract available.
References
-
- Lankisch P.G. Natural course of chronic pancreatitis. Pancreatology. 2001;1:3–14. - PubMed
-
- Adams D.B., Davis B.R., Anderson M.C. Colonic complications of pancreatitis. Am. Surg. 1994;60:44–49. - PubMed
-
- Etemad B., Whitcomb D.C. Chronic pancreatitis: diagnosis, classification, and new genetic developments. Gastroenterology. 2001;120:682–707. - PubMed
-
- Tieftrunk E., Demir I.E., Simon P., Friess H., Ceyhan G.O. Evidence of pancreatic neuropathy and neuropathic pain in hereditary chronic pancreatitis. Pancreatology. 2013;13:629–630. - PubMed
-
- Marks I.N., Bank S. The aetiology, clinical features and diagnosis of pancreatitis in the South Western Cape; a review of 243 cases. S. Afr. Med. J. 1963;37:1039–1053. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

