Glycine receptor α3 and α2 subunits mediate tonic and exogenous agonist-induced currents in forebrain
- PMID: 28784756
- PMCID: PMC5576794
- DOI: 10.1073/pnas.1703839114
Glycine receptor α3 and α2 subunits mediate tonic and exogenous agonist-induced currents in forebrain
Abstract
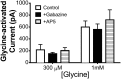
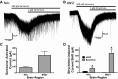
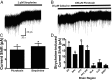
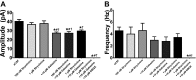
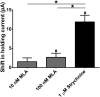
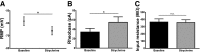
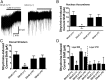
Neuronal inhibition can occur via synaptic mechanisms or through tonic activation of extrasynaptic receptors. In spinal cord, glycine mediates synaptic inhibition through the activation of heteromeric glycine receptors (GlyRs) composed primarily of α1 and β subunits. Inhibitory GlyRs are also found throughout the brain, where GlyR α2 and α3 subunit expression exceeds that of α1, particularly in forebrain structures, and coassembly of these α subunits with the β subunit appears to occur to a lesser extent than in spinal cord. Here, we analyzed GlyR currents in several regions of the adolescent mouse forebrain (striatum, prefrontal cortex, hippocampus, amygdala, and bed nucleus of the stria terminalis). Our results show ubiquitous expression of GlyRs that mediate large-amplitude currents in response to exogenously applied glycine in these forebrain structures. Additionally, tonic inward currents were also detected, but only in the striatum, hippocampus, and prefrontal cortex (PFC). These tonic currents were sensitive to both strychnine and picrotoxin, indicating that they are mediated by extrasynaptic homomeric GlyRs. Recordings from mice deficient in the GlyR α3 subunit (Glra3-/-) revealed a lack of tonic GlyR currents in the striatum and the PFC. In Glra2-/Y animals, GlyR tonic currents were preserved; however, the amplitudes of current responses to exogenous glycine were significantly reduced. We conclude that functional α2 and α3 GlyRs are present in various regions of the forebrain and that α3 GlyRs specifically participate in tonic inhibition in the striatum and PFC. Our findings suggest roles for glycine in regulating neuronal excitability in the forebrain.
Keywords: Cys-loop receptor; alpha subunits; glycine receptor; strychnine; tonic inhibition.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
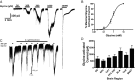
Similar articles
-
Pharmacological characterization of glycine-gated chloride currents recorded in rat hippocampal slices.J Neurophysiol. 2002 Mar;87(3):1515-25. doi: 10.1152/jn.00365.2001. J Neurophysiol. 2002. PMID: 11877523
-
Strychnine-sensitive glycine receptors on pyramidal neurons in layers II/III of the mouse prefrontal cortex are tonically activated.J Neurophysiol. 2014 Sep 1;112(5):1169-78. doi: 10.1152/jn.00714.2013. Epub 2014 May 28. J Neurophysiol. 2014. PMID: 24872538 Free PMC article.
-
Distinct physiological mechanisms underlie altered glycinergic synaptic transmission in the murine mutants spastic, spasmodic, and oscillator.J Neurosci. 2006 May 3;26(18):4880-90. doi: 10.1523/JNEUROSCI.3991-05.2006. J Neurosci. 2006. PMID: 16672662 Free PMC article.
-
Glycine Receptor Drug Discovery.Adv Pharmacol. 2017;79:225-253. doi: 10.1016/bs.apha.2017.01.003. Epub 2017 Mar 21. Adv Pharmacol. 2017. PMID: 28528670 Review.
-
Molecular pharmacology of the glycine receptor chloride channel.Curr Pharm Des. 2007;13(23):2350-67. doi: 10.2174/138161207781368693. Curr Pharm Des. 2007. PMID: 17692006 Review.
Cited by
-
1H-NMR-Based Metabolomics in Autism Spectrum Disorder and Pediatric Acute-Onset Neuropsychiatric Syndrome.J Clin Med. 2022 Nov 1;11(21):6493. doi: 10.3390/jcm11216493. J Clin Med. 2022. PMID: 36362721 Free PMC article.
-
Glycine Promotes the Survival of a Subpopulation of Neural Stem Cells.Front Cell Dev Biol. 2018 Jul 11;6:68. doi: 10.3389/fcell.2018.00068. eCollection 2018. Front Cell Dev Biol. 2018. PMID: 30050902 Free PMC article.
-
Influence of nonsynaptic α1 glycine receptors on ethanol consumption and place preference.Addict Biol. 2020 Mar;25(2):e12726. doi: 10.1111/adb.12726. Epub 2019 Mar 18. Addict Biol. 2020. PMID: 30884072 Free PMC article.
-
The emerging role of glycine receptor α2 subunit defects in neurodevelopmental disorders.Front Mol Neurosci. 2025 Feb 11;18:1550863. doi: 10.3389/fnmol.2025.1550863. eCollection 2025. Front Mol Neurosci. 2025. PMID: 40007572 Free PMC article. Review.
-
Psychoactive substances: novel molecular insights and therapeutic potential for Alzheimer's disease.Transl Neurodegener. 2025 Jul 25;14(1):38. doi: 10.1186/s40035-025-00498-1. Transl Neurodegener. 2025. PMID: 40713680 Free PMC article. Review.
References
-
- Klausberger T, et al. Spike timing of dendrite-targeting bistratified cells during hippocampal network oscillations in vivo. Nat Neurosci. 2004;7:41–47. - PubMed
-
- Farrant M, Nusser Z. Variations on an inhibitory theme: Phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. - PubMed
-
- Mitchell SJ, Silver RA. Shunting inhibition modulates neuronal gain during synaptic excitation. Neuron. 2003;38:433–445. - PubMed
-
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABA A receptors: Modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

