Chaperonin GroEL accelerates protofibril formation and decorates fibrils of the Het-s prion protein
- PMID: 28784759
- PMCID: PMC5576843
- DOI: 10.1073/pnas.1711645114
Chaperonin GroEL accelerates protofibril formation and decorates fibrils of the Het-s prion protein
Abstract
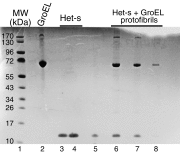
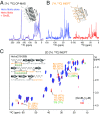
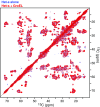
We have studied the interaction of the prototypical chaperonin GroEL with the prion domain of the Het-s protein using solution and solid-state NMR, electron and atomic force microscopies, and EPR. While GroEL accelerates Het-s protofibril formation by several orders of magnitude, the rate of appearance of fibrils is reduced. GroEL remains bound to Het-s throughout the aggregation process and densely decorates the fibrils at a regular spacing of ∼200 Å. GroEL binds to the Het-s fibrils via its apical domain located at the top of the large open ring. Thus, apo GroEL and bullet-shaped GroEL/GroES complexes in which only a single ring is capped by GroES interact with the Het-s fibrils; no evidence is seen for any interaction with football-shaped GroEL/GroES complexes in which both rings are capped by GroES. EPR spectroscopy shows that rotational motion of a nitroxide spin label, placed at the N-terminal end of the first β-strand of Het-s fibrils, is significantly reduced in both Het-s/GroEL aggregates and Het-s fibrils, but virtually completely eliminated in Het-s/GroEL fibrils, suggesting that in the latter, GroEL may come into close proximity to the nitroxide label. Solid-state NMR measurements indicate that GroEL binds to the mobile regions of the Het-s fibril comprising the N-terminal tail and a loop connecting β-strands 4 and 5, consistent with interactions involving GroEL binding consensus sequences located therein.
Keywords: EPR; NMR; amyloid–chaperone interactions; atomic force microscopy; electron microscopy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures












Similar articles
-
Suppression of amyloid fibrils using the GroEL apical domain.Sci Rep. 2016 Aug 4;6:31041. doi: 10.1038/srep31041. Sci Rep. 2016. PMID: 27488469 Free PMC article.
-
Characterisation of mutations in GroES that allow GroEL to function as a single ring.FEBS Lett. 2009 Jul 21;583(14):2365-71. doi: 10.1016/j.febslet.2009.06.027. Epub 2009 Jun 21. FEBS Lett. 2009. PMID: 19545569
-
Reaction Cycle of Chaperonin GroEL via Symmetric "Football" Intermediate.J Mol Biol. 2015 Sep 11;427(18):2912-8. doi: 10.1016/j.jmb.2015.04.007. Epub 2015 Apr 18. J Mol Biol. 2015. PMID: 25900372 Review.
-
Football- and bullet-shaped GroEL-GroES complexes coexist during the reaction cycle.J Biol Chem. 2008 Aug 29;283(35):23765-73. doi: 10.1074/jbc.M802541200. Epub 2008 Jun 20. J Biol Chem. 2008. PMID: 18567585 Free PMC article.
-
The GroEL-GroES Chaperonin Machine: A Nano-Cage for Protein Folding.Trends Biochem Sci. 2016 Jan;41(1):62-76. doi: 10.1016/j.tibs.2015.07.009. Epub 2015 Sep 25. Trends Biochem Sci. 2016. PMID: 26422689 Review.
Cited by
-
Large Chaperone Complexes Through the Lens of Nuclear Magnetic Resonance Spectroscopy.Annu Rev Biophys. 2022 May 9;51:223-246. doi: 10.1146/annurev-biophys-090921-120150. Epub 2022 Jan 19. Annu Rev Biophys. 2022. PMID: 35044800 Free PMC article. Review.
-
The expanding amyloid family: Structure, stability, function, and pathogenesis.Cell. 2021 Sep 16;184(19):4857-4873. doi: 10.1016/j.cell.2021.08.013. Cell. 2021. PMID: 34534463 Free PMC article. Review.
-
Phage phiKZ-The First of Giants.Viruses. 2021 Jan 20;13(2):149. doi: 10.3390/v13020149. Viruses. 2021. PMID: 33498475 Free PMC article. Review.
-
What are the 100 most cited fungal genera?Stud Mycol. 2024 Jul;108:1-411. doi: 10.3114/sim.2024.108.01. Epub 2024 Jul 15. Stud Mycol. 2024. PMID: 39100921 Free PMC article.
-
Oligomerization of a molecular chaperone modulates its activity.Elife. 2018 May 1;7:e35731. doi: 10.7554/eLife.35731. Elife. 2018. PMID: 29714686 Free PMC article.
References
-
- Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: From nascent chain to folded protein. Science. 2002;295:1852–1858. - PubMed
-
- Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. - PubMed
-
- Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475:324–332. - PubMed
-
- Wyatt AR, Yerbury JJ, Dabbs RA, Wilson MR. Roles of extracellular chaperones in amyloidosis. J Mol Biol. 2012;421:499–516. - PubMed
-
- Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

