Aquaporins facilitate hydrogen peroxide entry into guard cells to mediate ABA- and pathogen-triggered stomatal closure
- PMID: 28784763
- PMCID: PMC5576802
- DOI: 10.1073/pnas.1704754114
Aquaporins facilitate hydrogen peroxide entry into guard cells to mediate ABA- and pathogen-triggered stomatal closure
Abstract
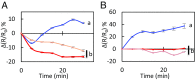
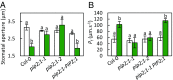
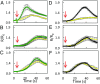
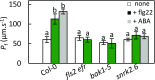
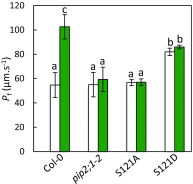
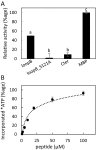
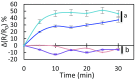
Stomatal movements are crucial for the control of plant water status and protection against pathogens. Assays on epidermal peels revealed that, similar to abscisic acid (ABA), pathogen-associated molecular pattern (PAMP) flg22 requires the AtPIP2;1 aquaporin to induce stomatal closure. Flg22 also induced an increase in osmotic water permeability (Pf) of guard cell protoplasts through activation of AtPIP2;1. The use of HyPer, a genetic probe for intracellular hydrogen peroxide (H2O2), revealed that both ABA and flg22 triggered an accumulation of H2O2 in wild-type but not pip2;1 guard cells. Pretreatment of guard cells with flg22 or ABA facilitated the influx of exogenous H2O2 Brassinosteroid insensitive 1-associated receptor kinase 1 (BAK1) and open stomata 1 (OST1)/Snf1-related protein kinase 2.6 (SnRK2.6) were both necessary to flg22-induced Pf and both phosphorylated AtPIP2;1 on Ser121 in vitro. Accumulation of H2O2 and stomatal closure as induced by flg22 was restored in pip2;1 guard cells by a phosphomimetic form (Ser121Asp) but not by a phosphodeficient form (Ser121Ala) of AtPIP2;1. We propose a mechanism whereby phosphorylation of AtPIP2;1 Ser121 by BAK1 and/or OST1 is triggered in response to flg22 to activate its water and H2O2 transport activities. This work establishes a signaling role of plasma membrane aquaporins in guard cells and potentially in other cellular context involving H2O2 signaling.
Keywords: aquaporin; guard cell signaling; hydrogen peroxide; pathogen; stomatal movement.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Hetherington AM, Woodward FI. The role of stomata in sensing and driving environmental change. Nature. 2003;424:901–908. - PubMed
-
- Roelfsema MR, Hedrich R. In the light of stomatal opening: New insights into ‘the Watergate’. New Phytol. 2005;167:665–691. - PubMed
-
- Joshi-Saha A, Valon C, Leung J. A brand new START: Abscisic acid perception and transduction in the guard cell. Sci Signal. 2011;4:re4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

