Structural insights into the catalytic mechanism of a sacrificial sulfur insertase of the N-type ATP pyrophosphatase family, LarE
- PMID: 28784764
- PMCID: PMC5576804
- DOI: 10.1073/pnas.1704967114
Structural insights into the catalytic mechanism of a sacrificial sulfur insertase of the N-type ATP pyrophosphatase family, LarE
Abstract
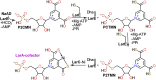
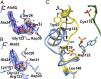
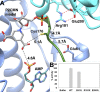
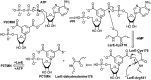
The lar operon in Lactobacillus plantarum encodes five Lar proteins (LarA/B/C/D/E) that collaboratively synthesize and incorporate a niacin-derived Ni-containing cofactor into LarA, an Ni-dependent lactate racemase. Previous studies have established that two molecules of LarE catalyze successive thiolation reactions by donating the sulfur atom of their exclusive cysteine residues to the substrate. However, the catalytic mechanism of this very unusual sulfur-sacrificing reaction remains elusive. In this work, we present the crystal structures of LarE in ligand-free and several ligand-bound forms, demonstrating that LarE is a member of the N-type ATP pyrophosphatase (PPase) family with a conserved N-terminal ATP PPase domain and a unique C-terminal domain harboring the putative catalytic site. Structural analysis, combined with structure-guided mutagenesis, leads us to propose a catalytic mechanism that establishes LarE as a paradigm for sulfur transfer through sacrificing its catalytic cysteine residue.
Keywords: ATP pyrophosphatase; Lar protein; catalysis; crystal structure; thiolation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
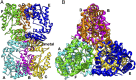

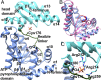

References
-
- Desguin B, et al. METALLOPROTEINS. A tethered niacin-derived pincer complex with a nickel-carbon bond in lactate racemase. Science. 2015;349:66–69. - PubMed
-
- Zhang X, Chung LW. Alternative mechanistic strategy for enzyme catalysis in a Ni-dependent lactate racemase (LarA): Intermediate destabilization by the cofactor. Chemistry. 2017;23:3623–3630. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

