Phosphorylation of serine96 of histidine-rich calcium-binding protein by the Fam20C kinase functions to prevent cardiac arrhythmia
- PMID: 28784772
- PMCID: PMC5576816
- DOI: 10.1073/pnas.1706441114
Phosphorylation of serine96 of histidine-rich calcium-binding protein by the Fam20C kinase functions to prevent cardiac arrhythmia
Abstract
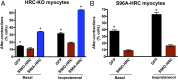
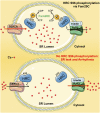
Precise Ca cycling through the sarcoplasmic reticulum (SR), a Ca storage organelle, is critical for proper cardiac muscle function. This cycling initially involves SR release of Ca via the ryanodine receptor, which is regulated by its interacting proteins junctin and triadin. The sarco/endoplasmic reticulum Ca ATPase (SERCA) pump then refills SR Ca stores. Histidine-rich Ca-binding protein (HRC) resides in the lumen of the SR, where it contributes to the regulation of Ca cycling by protecting stressed or failing hearts. The common Ser96Ala human genetic variant of HRC strongly correlates with life-threatening ventricular arrhythmias in patients with idiopathic dilated cardiomyopathy. However, the underlying molecular pathways of this disease remain undefined. Here, we demonstrate that family with sequence similarity 20C (Fam20C), a recently characterized protein kinase in the secretory pathway, phosphorylates HRC on Ser96. HRC Ser96 phosphorylation was confirmed in cells and human hearts. Furthermore, a Ser96Asp HRC variant, which mimics constitutive phosphorylation of Ser96, diminished delayed aftercontractions in HRC null cardiac myocytes. This HRC phosphomimetic variant was also able to rescue the aftercontractions elicited by the Ser96Ala variant, demonstrating that phosphorylation of Ser96 is critical for the cardioprotective function of HRC. Phosphorylation of HRC on Ser96 regulated the interactions of HRC with both triadin and SERCA2a, suggesting a unique mechanism for regulation of SR Ca homeostasis. This demonstration of the role of Fam20C-dependent phosphorylation in heart disease will open new avenues for potential therapeutic approaches against arrhythmias.
Keywords: Fam20C kinase; arrhythmia; heart; histidine-rich calcium-binding protein; phosphorylation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Abnormal calcium cycling and cardiac arrhythmias associated with the human Ser96Ala genetic variant of histidine-rich calcium-binding protein.J Am Heart Assoc. 2013 Oct 14;2(5):e000460. doi: 10.1161/JAHA.113.000460. J Am Heart Assoc. 2013. PMID: 24125847 Free PMC article.
-
Impaired calcium homeostasis is associated with sudden cardiac death and arrhythmias in a genetic equivalent mouse model of the human HRC-Ser96Ala variant.Cardiovasc Res. 2017 Sep 1;113(11):1403-1417. doi: 10.1093/cvr/cvx113. Cardiovasc Res. 2017. PMID: 28859293
-
Histidine-rich Ca-binding protein interacts with sarcoplasmic reticulum Ca-ATPase.Am J Physiol Heart Circ Physiol. 2007 Sep;293(3):H1581-9. doi: 10.1152/ajpheart.00278.2007. Epub 2007 May 25. Am J Physiol Heart Circ Physiol. 2007. PMID: 17526652
-
The Histidine-Rich Calcium Binding Protein in Regulation of Cardiac Rhythmicity.Front Physiol. 2018 Sep 27;9:1379. doi: 10.3389/fphys.2018.01379. eCollection 2018. Front Physiol. 2018. PMID: 30319456 Free PMC article. Review.
-
Histidine-rich calcium binding protein: the new regulator of sarcoplasmic reticulum calcium cycling.J Mol Cell Cardiol. 2011 Jan;50(1):43-9. doi: 10.1016/j.yjmcc.2010.08.021. Epub 2010 Aug 31. J Mol Cell Cardiol. 2011. PMID: 20807542 Free PMC article. Review.
Cited by
-
Thinking outside of the cell: Secreted protein kinases in bacteria, parasites, and mammals.IUBMB Life. 2019 Jun;71(6):749-759. doi: 10.1002/iub.2040. Epub 2019 Apr 2. IUBMB Life. 2019. PMID: 30941842 Free PMC article. Review.
-
Ser96Ala genetic variant of the human histidine-rich calcium-binding protein is a genetic predictor of recurrence after catheter ablation in patients with paroxysmal atrial fibrillation.PLoS One. 2019 Mar 6;14(3):e0213208. doi: 10.1371/journal.pone.0213208. eCollection 2019. PLoS One. 2019. PMID: 30840693 Free PMC article.
-
FAM20C: A key protein kinase in multiple diseases.Genes Dis. 2023 Nov 23;12(2):101179. doi: 10.1016/j.gendis.2023.101179. eCollection 2025 Mar. Genes Dis. 2023. PMID: 39790934 Free PMC article. Review.
-
The ABCs of the atypical Fam20 secretory pathway kinases.J Biol Chem. 2021 Jan-Jun;296:100267. doi: 10.1016/j.jbc.2021.100267. Epub 2021 Jan 8. J Biol Chem. 2021. PMID: 33759783 Free PMC article. Review.
-
Integrated Quantitative Phosphoproteomics and Cell-based Functional Screening Reveals Specific Pathological Cardiac Hypertrophy-related Phosphorylation Sites.Mol Cells. 2021 Jul 31;44(7):500-516. doi: 10.14348/molcells.2021.4002. Mol Cells. 2021. PMID: 34158421 Free PMC article.
References
-
- Hershberger RE, Hedges DJ, Morales A. Dilated cardiomyopathy: The complexity of a diverse genetic architecture. Nat Rev Cardiol. 2013;10:531–547. - PubMed
-
- Zipes DP, Wellens HJ. Sudden cardiac death. Circulation. 1998;98:2334–2351. - PubMed
-
- Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

