The effects of heat stress on morphological properties and intracellular signaling of denervated and intact soleus muscles in rats
- PMID: 28784851
- PMCID: PMC5555886
- DOI: 10.14814/phy2.13350
The effects of heat stress on morphological properties and intracellular signaling of denervated and intact soleus muscles in rats
Abstract
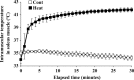
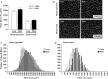
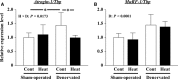
The effects of heat stress on the morphological properties and intracellular signaling of innervated and denervated soleus muscles were investigated. Heat stress was applied to rats by immersing their hindlimbs in a warm water bath (42°C, 30 min/day, every other day following unilateral denervation) under anesthesia. During 14 days of experimental period, heat stress for a total of seven times promoted growth-related hypertrophy in sham-operated muscles and attenuated atrophy in denervated muscles. In denervated muscles, the transcription of ubiquitin ligase, atrogin-1/muscle atrophy F-box (Atrogin-1), and muscle RING-finger protein-1 (MuRF-1), genes was upregulated and ubiquitination of proteins was also increased. Intermittent heat stress inhibited the upregulation of Atrogin-1, but not MuRF-1 transcription. And the denervation-caused reduction in phosphorylated protein kinase B (Akt), 70-kDa heat-shock protein (HSP70), and peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α), which are negative regulators of Atrogin-1 and MuRF-1 transcription, was mitigated. In sham-operated muscles, repeated application of heat stress did not affect Atrogin-1 and MuRF-1 transcription, but increased the level of phosphorylated Akt and HSP70, but not PGC-1α Furthermore, the phosphorylation of Akt and ribosomal protein S6, which is known to stimulate protein synthesis, was increased immediately after a single heat stress particularly in the sham-operated muscles. The effect of a heat stress was suppressed in denervated muscles. These results indicated that the beneficial effects of heat stress on the morphological properties of muscles were brought regardless of innervation. However, the responses of intracellular signaling to heat stress were distinct between the innervated and denervated muscles.
Keywords: Atrophy; heat stress; hypertrophy; neural innervation; skeletal muscle.
© 2017 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of The Physiological Society and the American Physiological Society.
Figures




Similar articles
-
Mechanisms involved in 3',5'-cyclic adenosine monophosphate-mediated inhibition of the ubiquitin-proteasome system in skeletal muscle.Endocrinology. 2009 Dec;150(12):5395-404. doi: 10.1210/en.2009-0428. Epub 2009 Oct 16. Endocrinology. 2009. PMID: 19837877
-
Clenbuterol suppresses proteasomal and lysosomal proteolysis and atrophy-related genes in denervated rat soleus muscles independently of Akt.Am J Physiol Endocrinol Metab. 2012 Jan 1;302(1):E123-33. doi: 10.1152/ajpendo.00188.2011. Epub 2011 Sep 27. Am J Physiol Endocrinol Metab. 2012. PMID: 21952035
-
Role of ubiquitin-proteasome proteolysis in muscle fiber destruction in experimental chloroquine-induced myopathy.Muscle Nerve. 2009 Apr;39(4):521-8. doi: 10.1002/mus.21223. Muscle Nerve. 2009. PMID: 19296457
-
Atrogin-1, MuRF-1, and sarcopenia.Endocrine. 2013 Feb;43(1):12-21. doi: 10.1007/s12020-012-9751-7. Epub 2012 Jul 20. Endocrine. 2013. PMID: 22815045 Free PMC article. Review.
-
The role of E3 ubiquitin-ligases MuRF-1 and MAFbx in loss of skeletal muscle mass.Free Radic Biol Med. 2016 Sep;98:218-230. doi: 10.1016/j.freeradbiomed.2015.12.031. Epub 2015 Dec 29. Free Radic Biol Med. 2016. PMID: 26738803 Review.
Cited by
-
The impact of heat therapy on neuromuscular function and muscle atrophy in diabetic rats.Front Physiol. 2023 Jan 5;13:1039588. doi: 10.3389/fphys.2022.1039588. eCollection 2022. Front Physiol. 2023. PMID: 36685197 Free PMC article.
-
Effects of Twelve Sessions of High-Temperature Sauna Baths on Body Composition in Healthy Young Men.Int J Environ Res Public Health. 2021 Apr 22;18(9):4458. doi: 10.3390/ijerph18094458. Int J Environ Res Public Health. 2021. PMID: 33922289 Free PMC article.
-
Heat therapy improves body composition and muscle function but does not affect capillary or collateral growth in a model of obesity and hindlimb ischemia.J Appl Physiol (1985). 2021 Feb 1;130(2):355-368. doi: 10.1152/japplphysiol.00535.2020. Epub 2020 Nov 12. J Appl Physiol (1985). 2021. PMID: 33180645 Free PMC article.
-
Skeletal muscle adaptations to heat therapy.J Appl Physiol (1985). 2020 Jun 1;128(6):1635-1642. doi: 10.1152/japplphysiol.00061.2020. Epub 2020 Apr 30. J Appl Physiol (1985). 2020. PMID: 32352340 Free PMC article.
-
Effects of home-based leg heat therapy on walking performance in patients with symptomatic peripheral artery disease: a pilot randomized trial.J Appl Physiol (1985). 2022 Sep 1;133(3):546-560. doi: 10.1152/japplphysiol.00143.2022. Epub 2022 Jun 30. J Appl Physiol (1985). 2022. PMID: 35771219 Free PMC article. Clinical Trial.
References
-
- Adhihetty, P. J. , Uguccioni G., Leick L., Hidalgo J., Pilegaard H., and Hood D. A.. 2009. The role of PGC‐1alpha on mitochondrial function and apoptotic susceptibility in muscle. Am. J. Physiol. Cell Physiol. 297:C217–C225. - PubMed
-
- Bodine, S. C. , Latres E., Baumhueter S., Lai V. K., Nunez L., Clarke B. A., et al. 2001a. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294:1704–1708. - PubMed
-
- Bodine, S. C. , Stitt T. N., Gonzalez M., Kline W. O., Stover G. L., Bauerlein R., et al. 2001b. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol. 3:1014–1019. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

