Mapping sulphadoxine-pyrimethamine-resistant Plasmodium falciparum malaria in infected humans and in parasite populations in Africa
- PMID: 28785011
- PMCID: PMC5547055
- DOI: 10.1038/s41598-017-06708-9
Mapping sulphadoxine-pyrimethamine-resistant Plasmodium falciparum malaria in infected humans and in parasite populations in Africa
Abstract
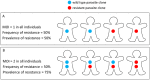
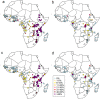
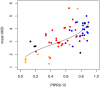
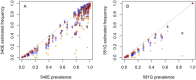
Intermittent preventive treatment (IPT) with sulphadoxine-pyrimethamine in vulnerable populations reduces malaria morbidity in Africa, but resistance mutations in the parasite dhps gene (combined with dhfr mutations) threaten its efficacy. We update a systematic review to map the prevalence of K540E and A581G mutations in 294 surveys of infected humans across Africa from 2004-present. Interpreting these data is complicated by multiclonal infections in humans, especially in high transmission areas. We extend statistical methods to estimate the frequency, i.e. the proportion of resistant clones in the parasite population at each location, and so standardise for varying transmission levels. Both K540E and A581G mutations increased in prevalence and frequency in 60% of areas after 2008, highlighting the need for ongoing surveillance. Resistance measures within countries were similar within 300 km, suggesting an appropriate spatial scale for surveillance. Spread of the mutations tended to accelerate once their prevalence exceeded 10% (prior to fixation). Frequencies of resistance in parasite populations are the same or lower than prevalence in humans, so more areas would be classified as likely to benefit from IPT if similar frequency thresholds were applied. We propose that the use of resistance frequencies as well as prevalence measures for policy decisions should be evaluated.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

