Molecular and Physical Mechanisms of Fibrinolysis and Thrombolysis from Mathematical Modeling and Experiments
- PMID: 28785035
- PMCID: PMC5547096
- DOI: 10.1038/s41598-017-06383-w
Molecular and Physical Mechanisms of Fibrinolysis and Thrombolysis from Mathematical Modeling and Experiments
Abstract
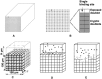
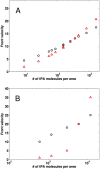
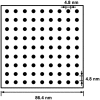
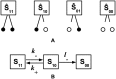
Despite the common use of thrombolytic drugs, especially in stroke treatment, there are many conflicting studies on factors affecting fibrinolysis. Because of the complexity of the fibrinolytic system, mathematical models closely tied with experiments can be used to understand relationships within the system. When tPA is introduced at the clot or thrombus edge, lysis proceeds as a front. We developed a multiscale model of fibrinolysis that includes the main chemical reactions: the microscale model represents a single fiber cross-section; the macroscale model represents a three-dimensional fibrin clot. The model successfully simulates the spatial and temporal locations of all components and elucidates how lysis rates are determined by the interplay between the number of tPA molecules in the system and clot structure. We used the model to identify kinetic conditions necessary for fibrinolysis to proceed as a front. We found that plasmin regulates the local concentration of tPA through forced unbinding via degradation of fibrin and tPA release. The mechanism of action of tPA is affected by the number of molecules present with respect to fibrin fibers. The physical mechanism of plasmin action (crawling) and avoidance of inhibition is defined. Many of these new findings have significant implications for thrombolytic treatment.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Mutch, N. J. & Booth, N. A. Plasminogen activation and regulation of fibrinolysis. In Marder, V. J. & et al. (eds) Hemostasis and Thrombosis: Basic Principles and Clinical Practice 314–333 (Lippincott Williams & Wilkins, Philadelphia, sixth edn 2013).
-
- Blinc A, Magdic J, Fric J, Musevic I. Atomic force microscopy of fibrin networks and plasma clots during fibrinolysis. Fibrinolysis Proteol. 2000;14:288–299. doi: 10.1054/fipr.2000.0085. - DOI
-
- Veklich Y, Francis CW, White J, Weisel JW. Structural studies of fibrinolysis by electron microscopy. Blood. 1998;92:4721–4729. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

