Generation of glyceraldehyde-derived advanced glycation end-products in pancreatic cancer cells and the potential of tumor promotion
- PMID: 28785145
- PMCID: PMC5526761
- DOI: 10.3748/wjg.v23.i27.4910
Generation of glyceraldehyde-derived advanced glycation end-products in pancreatic cancer cells and the potential of tumor promotion
Abstract
Aim: To determine the possibility that diabetes mellitus promotes pancreatic ductal adenocarcinoma via glyceraldehyde (GA)-derived advanced glycation-end products (GA-AGEs).
Methods: PANC-1, a human pancreatic cancer cell line, was treated with 1-4 mmol/L GA for 24 h. The cell viability and intracellular GA-AGEs were measured by WST-8 assay and slot blotting. Moreover, immunostaining of PANC-1 cells with an anti-GA-AGE antibody was performed. Western blotting (WB) was used to analyze the molecular weight of GA-AGEs. Heat shock proteins 90α, 90β, 70, 27 and cleaved caspase-3 were analyzed by WB. In addition, PANC-1 cells were treated with GA-AGEs-bovine serum albumin (GA-AGEs-BSA), as a model of extracellular GA-AGEs, and proliferation of PANC-1 cells was measured.
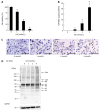
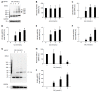
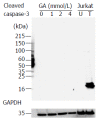
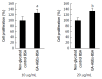
Results: In PANC-1 cells, GA induced the production of GA-AGEs and cell death in a dose-dependent manner. PANC-1 cell viability was approximately 40% with a 2 mmol/L GA treatment and decreased to almost 0% with a 4 mmol/L GA treatment (each significant difference was P < 0.01). Cells treated with 2 and 4 mmol/L GA produced 6.4 and 21.2 μg/mg protein of GA-AGEs, respectively (P < 0.05 and P < 0.01). The dose-dependent production of some high-molecular-weight (HMW) complexes of HSP90β, HSP70, and HSP27 was observed following administration of GA. We considered HMW complexes to be dimers and trimers with GA-AGEs-mediated aggregation. Cleaved caspase-3 could not be detected with WB. Furthermore, 10 and 20 μg/mL GA-AGEs-BSA was 27% and 34% greater than that of control cells, respectively (P < 0.05 and P < 0.01).
Conclusion: Although intracellular GA-AGEs induce pancreatic cancer cell death, their secretion and release may promote the proliferation of other pancreatic cancer cells.
Keywords: Glyceraldehyde-derived advanced glycation-end products; Pancreatic ductal adenocarcinoma; Tumor promotion.
Conflict of interest statement
Conflict-of-interest statement: The authors declare no competing financial interests.
Figures

Similar articles
-
Structures of Toxic Advanced Glycation End-Products Derived from Glyceraldehyde, A Sugar Metabolite.Biomolecules. 2024 Feb 8;14(2):202. doi: 10.3390/biom14020202. Biomolecules. 2024. PMID: 38397439 Free PMC article. Review.
-
Proteomic Investigation of Glyceraldehyde-Derived Intracellular AGEs and Their Potential Influence on Pancreatic Ductal Cells.Cells. 2021 Apr 24;10(5):1005. doi: 10.3390/cells10051005. Cells. 2021. PMID: 33923186 Free PMC article.
-
The formation of intracellular glyceraldehyde-derived advanced glycation end-products and cytotoxicity.J Gastroenterol. 2010 Jun;45(6):646-55. doi: 10.1007/s00535-009-0193-9. Epub 2010 Jan 19. J Gastroenterol. 2010. PMID: 20084527
-
Impact of intracellular glyceraldehyde-derived advanced glycation end-products on human hepatocyte cell death.Sci Rep. 2017 Oct 27;7(1):14282. doi: 10.1038/s41598-017-14711-3. Sci Rep. 2017. PMID: 29079763 Free PMC article.
-
Advanced glycation end products (AGEs) and cardiovascular dysfunction: focus on high molecular weight AGEs.Amino Acids. 2017 Sep;49(9):1535-1541. doi: 10.1007/s00726-017-2464-8. Epub 2017 Jul 14. Amino Acids. 2017. PMID: 28710551 Review.
Cited by
-
Intracellular toxic advanced glycation end-products in cardiomyocytes may cause cardiovascular disease.Sci Rep. 2019 Feb 14;9(1):2121. doi: 10.1038/s41598-019-39202-5. Sci Rep. 2019. PMID: 30765817 Free PMC article.
-
Novel In Vitro Assay of the Effects of Kampo Medicines against Intra/Extracellular Advanced Glycation End-Products in Oral, Esophageal, and Gastric Epithelial Cells.Metabolites. 2023 Jul 24;13(7):878. doi: 10.3390/metabo13070878. Metabolites. 2023. PMID: 37512585 Free PMC article.
-
Potential of an Interorgan Network Mediated by Toxic Advanced Glycation End-Products in a Rat Model.Nutrients. 2020 Dec 29;13(1):80. doi: 10.3390/nu13010080. Nutrients. 2020. PMID: 33383715 Free PMC article.
-
Intracellular Toxic AGEs (TAGE) Triggers Numerous Types of Cell Damage.Biomolecules. 2021 Mar 5;11(3):387. doi: 10.3390/biom11030387. Biomolecules. 2021. PMID: 33808036 Free PMC article. Review.
-
Structures of Toxic Advanced Glycation End-Products Derived from Glyceraldehyde, A Sugar Metabolite.Biomolecules. 2024 Feb 8;14(2):202. doi: 10.3390/biom14020202. Biomolecules. 2024. PMID: 38397439 Free PMC article. Review.
References
-
- Shimasaki T, Yamamoto S, Ishigaki Y, Takata T, Arisawa T, Motoo Y, Tomosug N, Minamoto T. Identification and functional analysis of an EMT-accelerating factor induced in pancreatic cancer cells by anticancer agent. Suizo. 2016;31:76–84.
-
- Wada K, Takaori K, Traverso LW, Hruban RH, Furukawa T, Brentnall TA, Hatori T, Sano K, Takada T, Majima Y, et al. Clinical importance of Familial Pancreatic Cancer Registry in Japan: a report from kick-off meeting at International Symposium on Pancreas Cancer 2012. J Hepatobiliary Pancreat Sci. 2013;20:557–566. - PubMed
-
- Bobrowski A, Spitzner M, Bethge S, Mueller-Graf F, Vollmar B, Zechner D. Risk factors for pancreatic ductal adenocarcinoma specifically stimulate pancreatic duct glands in mice. Am J Pathol. 2013;182:965–974. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

