Translating epigenetics into clinic: focus on lupus
- PMID: 28785369
- PMCID: PMC5541721
- DOI: 10.1186/s13148-017-0378-7
Translating epigenetics into clinic: focus on lupus
Abstract
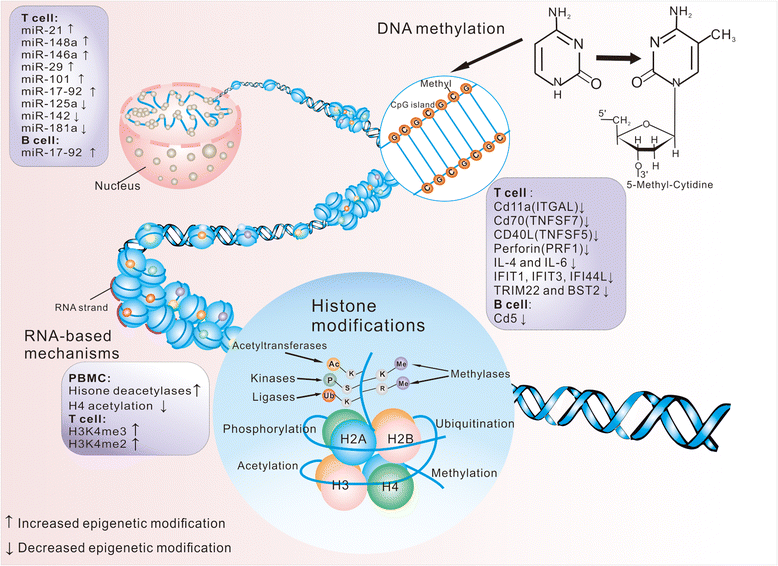
Systemic lupus erythematosus (SLE) is a chronic relapsing-remitting autoimmune disease with highly heterogeneous phenotypes. Biomarkers with high sensitivity and specificity are useful for early diagnosis as well as monitoring disease activity and long-term complications. Epigenetics potentially provide novel biomarkers in autoimmune diseases. These may include DNA methylation changes in relevant lupus-prone genes or histone modifications and microRNAs to upregulate and downregulate relevant gene expression. The timing and nature of epigenetic modification provide such changes. In lupus, DNA methylation alterations in cytokine genes, such as IFN-related gene and retrovirus gene, have been found to offer biomarkers for lupus diagnosis. Histone modifications such as histone methylation and acetylation lead to transcriptional alterations of several genes such as PTPN22, LRP1B, and TNFSF70. There are varieties of microRNAs applied as lupus biomarkers, including DNMT1-related microRNAs, renal function-associated microRNAs, microRNAs involved in the immune system, and microRNAs for phenotype classification. Thus, we conclude a wide range of promising roles of epigenetic biomarkers aiding in the diagnosing and monitoring of lupus diseases and the risk of organ damage.
Keywords: Biomarker; DNA methylation; Epigenetic modification; Histone modification; MicroRNAs; Systemic lupus erythematosus.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Epigenetic perspectives in systemic lupus erythematosus: pathogenesis, biomarkers, and therapeutic potentials.Clin Rev Allergy Immunol. 2010 Aug;39(1):3-9. doi: 10.1007/s12016-009-8165-7. Clin Rev Allergy Immunol. 2010. PMID: 19639427 Review.
-
[Epigenetic disturbances in systemic lupus erythematosus].Wiad Lek. 2018;71(1 pt 1):32-39. Wiad Lek. 2018. PMID: 29558349 Review. Polish.
-
The critical role of epigenetics in systemic lupus erythematosus and autoimmunity.J Autoimmun. 2016 Nov;74:118-138. doi: 10.1016/j.jaut.2016.06.020. Epub 2016 Jul 6. J Autoimmun. 2016. PMID: 27396525 Review.
-
Epigenetics and Systemic Lupus Erythematosus: Unmet Needs.Clin Rev Allergy Immunol. 2016 Jun;50(3):367-76. doi: 10.1007/s12016-015-8497-4. Clin Rev Allergy Immunol. 2016. PMID: 26206675 Review.
-
Genome-wide analysis of histone H3 lysine 4 trimethylation by ChIP-chip in peripheral blood mononuclear cells of systemic lupus erythematosus patients.Clin Exp Rheumatol. 2010 Mar-Apr;28(2):158-68. Epub 2010 May 13. Clin Exp Rheumatol. 2010. PMID: 20483040
Cited by
-
Identifying novel associations in GWAS by hierarchical Bayesian latent variable detection of differentially misclassified phenotypes.BMC Bioinformatics. 2020 May 7;21(1):178. doi: 10.1186/s12859-020-3387-z. BMC Bioinformatics. 2020. PMID: 32381021 Free PMC article.
-
Epigenetic Priming in Immunodeficiencies.Front Cell Dev Biol. 2019 Jul 10;7:125. doi: 10.3389/fcell.2019.00125. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31355198 Free PMC article. Review.
-
Epigenetic Mechanisms and Posttranslational Modifications in Systemic Lupus Erythematosus.Int J Mol Sci. 2019 Nov 13;20(22):5679. doi: 10.3390/ijms20225679. Int J Mol Sci. 2019. PMID: 31766160 Free PMC article. Review.
-
Systematics for types and effects of DNA variations.BMC Genomics. 2018 Dec 28;19(1):974. doi: 10.1186/s12864-018-5262-0. BMC Genomics. 2018. PMID: 30591019 Free PMC article. Review.
-
Advances in Traditional Chinese Medicine for Modulating DNA Methylation in the Treatment of Inflammatory Diseases.Int J Mol Sci. 2025 Jun 30;26(13):6331. doi: 10.3390/ijms26136331. Int J Mol Sci. 2025. PMID: 40650121 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

