Klotho/FGF23 Axis in Chronic Kidney Disease and Cardiovascular Disease
- PMID: 28785560
- PMCID: PMC5527179
- DOI: 10.1159/000452880
Klotho/FGF23 Axis in Chronic Kidney Disease and Cardiovascular Disease
Abstract
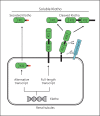
Background: Membrane αKlotho (hereinafter called Klotho) is highly expressed in the kidney and functions as a coreceptor of FGF receptors (FGFRs) to activate specific fibroblast growth factor 23 (FGF23) signal pathway. FGF23 is produced in bones and participates in the maintenance of mineral homeostasis. The extracellular domain of transmembrane Klotho can be cleaved by secretases and released into the circulation as soluble Klotho. Soluble Klotho does not only weakly activate FGFRs to transduce the FGF23 signaling pathway, but also functions as an enzyme and hormonal substance to play a variety of biological functions. FGF23 exerts its biological effects through activation of FGFRs in a Klotho-dependent manner. However, extremely high FGF23 can exert its pathological action in a Klotho-independent manner.
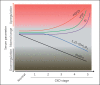
Summary: The decline in serum and urinary Klotho followed by a rise in serum FGF23 at an early stage of chronic kidney disease (CKD) functions as an early biomarker for kidney dysfunction and can also serve as a predictor for risk of cardiovascular disease (CVD) and mortality in both CKD patients and the general population. Moreover, Klotho deficiency is a pathogenic factor for CKD progression and CVD. FGF23 may also contribute to CVD. Prevention of Klotho decline, reactivation of endogenous Klotho production, or supplementation of exogenous Klotho attenuate renal fibrosis, retard CKD progression, improve mineral metabolism, ameliorate cardiomyopathy, and alleviate vascular calcification in CKD. However, the poor CVD outcome after depletion of FGF23 with FGF23 antibody stimulates the generation of a more specific inhibitor of FGF23 for CKD treatment.
Key message: Klotho/FGF23 may not only be diagnostic and/or prognostic biomarkers for CKD and CVD, but are also pathogenic contributors to CKD progression and CVD development. The Klotho/FGF23 axis should be a novel target for renal clinics.
Keywords: Biomarker; Cardiovascular disease; Chronic kidney disease; FGF23; Klotho.
Figures

References
-
- Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. - PubMed
-
- Ito S, Kinoshita S, Shiraishi N, et al. Molecular cloning and expression analyses of mouse betaklotho, which encodes a novel Klotho family protein. Mech Dev. 2000;98:115–119. - PubMed
-
- Ito S, Fujimori T, Hayashizaki Y, et al. Identification of a novel mouse membrane-bound family 1 glycosidase-like protein, which carries an atypical active site structure. Biochim Biophys Acta. 2002;1576:341–345. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

