Action Potential Dynamics in Fine Axons Probed with an Axonally Targeted Optical Voltage Sensor
- PMID: 28785728
- PMCID: PMC5526655
- DOI: 10.1523/ENEURO.0146-17.2017
Action Potential Dynamics in Fine Axons Probed with an Axonally Targeted Optical Voltage Sensor
Abstract
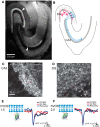
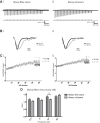
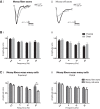
The complex and malleable conduction properties of axons determine how action potentials propagate through extensive axonal arbors to reach synaptic terminals. The excitability of axonal membranes plays a major role in neural circuit function, but because most axons are too thin for conventional electrical recording, their properties remain largely unexplored. To overcome this obstacle, we used a genetically encoded hybrid voltage sensor (hVOS) harboring an axonal targeting motif. Expressing this probe in transgenic mice enabled us to monitor voltage changes optically in two populations of axons in hippocampal slices, the large axons of dentate granule cells (mossy fibers) in the stratum lucidum of the CA3 region and the much finer axons of hilar mossy cells in the inner molecular layer of the dentate gyrus. Action potentials propagated with distinct velocities in each type of axon. Repetitive firing broadened action potentials in both populations, but at an intermediate frequency the degree of broadening differed. Repetitive firing also attenuated action potential amplitudes in both mossy cell and granule cell axons. These results indicate that the features of use-dependent action potential broadening, and possible failure, observed previously in large nerve terminals also appear in much finer unmyelinated axons. Subtle differences in the frequency dependences could influence the propagation of activity through different pathways to excite different populations of neurons. The axonally targeted hVOS probe used here opens up the diverse repertoire of neuronal processes to detailed biophysical study.
Keywords: Axonal membranes; excitability; fluorescence imaging; voltage imaging.
Conflict of interest statement
Authors report no conflict of interest.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
