Effect of temperature on FAD and NADH-derived signals and neurometabolic coupling in the mouse auditory and motor cortex
- PMID: 28785802
- PMCID: PMC5693747
- DOI: 10.1007/s00424-017-2037-4
Effect of temperature on FAD and NADH-derived signals and neurometabolic coupling in the mouse auditory and motor cortex
Abstract
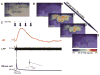
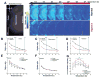
Tight coupling of neuronal metabolism to synaptic activity is critical to ensure that the supply of metabolic substrates meets the demands of neuronal signaling. Given the impact of temperature on metabolism, and the wide fluctuations of brain temperature observed during clinical hypothermia, we examined the effect of temperature on neurometabolic coupling. Intrinsic fluorescence signals of the oxidized form of flavin adenine dinucleotide (FAD) and the reduced form of nicotinamide adenine dinucleotide (NADH), and their ratios, were measured to assess neural metabolic state and local field potentials were recorded to measure synaptic activity in the mouse brain. Brain slice preparations were used to remove the potential impacts of blood flow. Tight coupling between metabolic signals and local field potential amplitudes was observed at a range of temperatures below 29 °C. However, above 29 °C, the metabolic and synaptic signatures diverged such that FAD signals were diminished, but local field potentials retained their amplitude. It was also observed that the declines in the FAD signals seen at high temperatures (and hence the decoupling between synaptic and metabolic events) are driven by low FAD availability at high temperatures. These data suggest that neurometabolic coupling, thought to be critical for ensuring the metabolic health of the brain, may show temperature dependence, and is related to temperature-dependent changes in FAD supplies.
Keywords: Auditory cortex; Flavoprotein imaging; Hippocampus; Hypothermia; Neurometabolic coupling; Temperature.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures





Similar articles
-
Ageing-related changes in GABAergic inhibition in mouse auditory cortex, measured using in vitro flavoprotein autofluorescence imaging.J Physiol. 2016 Jan 1;594(1):207-21. doi: 10.1113/JP271221. Epub 2015 Dec 14. J Physiol. 2016. PMID: 26503482 Free PMC article.
-
Changes in FAD and NADH fluorescence in neurosecretory terminals are triggered by calcium entry and by ADP production.J Membr Biol. 2005 Nov;208(2):113-24. doi: 10.1007/s00232-005-0824-x. J Membr Biol. 2005. PMID: 16645741
-
Measurement of mitochondrial NADH and FAD autofluorescence in live cells.Methods Mol Biol. 2015;1264:263-70. doi: 10.1007/978-1-4939-2257-4_23. Methods Mol Biol. 2015. PMID: 25631020
-
Intracellular coenzymes as natural biomarkers for metabolic activities and mitochondrial anomalies.Biomark Med. 2010 Apr;4(2):241-63. doi: 10.2217/bmm.10.1. Biomark Med. 2010. PMID: 20406068 Free PMC article. Review.
-
Differences in O2 availability resolve the apparent discrepancies in metabolic intrinsic optical signals in vivo and in vitro.Trends Neurosci. 2007 Aug;30(8):390-8. doi: 10.1016/j.tins.2007.06.001. Epub 2007 Jun 27. Trends Neurosci. 2007. PMID: 17590447 Free PMC article. Review.
Cited by
-
Flavin Adenine Dinucleotide Fluorescence as an Early Marker of Mitochondrial Impairment During Brain Hypoxia.Int J Mol Sci. 2020 Jun 1;21(11):3977. doi: 10.3390/ijms21113977. Int J Mol Sci. 2020. PMID: 32492921 Free PMC article.
-
Corticothalamic gating of population auditory thalamocortical transmission in mouse.Elife. 2021 May 24;10:e56645. doi: 10.7554/eLife.56645. Elife. 2021. PMID: 34028350 Free PMC article.
-
Fast autofluorescence imaging to evaluate dynamic changes in cell metabolism.J Biomed Opt. 2024 Dec;29(12):126501. doi: 10.1117/1.JBO.29.12.126501. Epub 2024 Dec 19. J Biomed Opt. 2024. PMID: 39703201 Free PMC article.
-
V-NeuroStack: Open-source 3D time stack software for identifying patterns in neuronal data.J Neurosci Res. 2023 Feb;101(2):217-231. doi: 10.1002/jnr.25139. Epub 2022 Oct 30. J Neurosci Res. 2023. PMID: 36309817 Free PMC article.
-
A novel dynamic network imaging analysis method reveals aging-related fragmentation of cortical networks in mouse.Netw Neurosci. 2021 Jun 21;5(2):569-590. doi: 10.1162/netn_a_00191. eCollection 2021. Netw Neurosci. 2021. PMID: 34189378 Free PMC article.
References
-
- Kowalska AMG, Szego D, Pineda AL, Ayala D, Xu Yuan, Hughes N, Tito A, Jabłonska J. THE THERMAL SCANNING FLUORESCENCE STUDY ON THE CONFORMATIONAL STABILITY OF GLUCOSE OXIDASE (GOD) FROM ASPERGILLUS NIGER. Food Chemistry and Biotechnology. 2007;71:35–48.
-
- Aihara H, Okada Y, Tamaki N. The effects of cooling and rewarming on the neuronal activity of pyramidal neurons in guinea pig hippocampal slices. Brain Res. 2001;893:36–45. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

