Incidence, Survival, and Treatment of Localized and Metastatic Neuroblastoma in Germany 1979-2015
- PMID: 28786082
- PMCID: PMC5680382
- DOI: 10.1007/s40272-017-0251-3
Incidence, Survival, and Treatment of Localized and Metastatic Neuroblastoma in Germany 1979-2015
Abstract
Background: A comprehensive clinical long-term survey over the complete spectrum of neuroblatoma disease is lacking in the literature.
Objective: Our objective was to describe the incidence, risk profiles, therapies, and outcomes for the total cohort of German patients with neuroblastoma including all clinical stages and risk groups.
Methods: Epidemiological, clinical, and outcome data of neuroblastoma patients who participated in one of the six consecutive national trials between 1979 and 2015 were analyzed retrospectively.
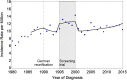
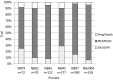
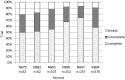
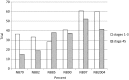
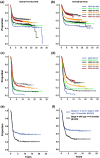
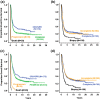
Results: Of all German neuroblastoma patients known to the national childhood cancer registry, ninety seven percent enrolled in one of the trials. The absolute neuroblastoma rate has increased slightly, whereas the median age at diagnosis has decreased. Except for the screening period (1995-2000), the risk factors lactate dehydrogenase (LDH), ferritin, chromosome 1p, and the MYCN oncogene have remained largely constant, with the exception of an increase in MYCN amplification at stage 4 for those aged ≥18 months between trials NB97 (27%) and NB2004 (35%). The 10-year overall survival increased in patients with stage 1-3 neuroblastoma from 83 to 91%, for stage 4S from 80 to 85%, and for stage 4 aged ≥18 months from 2 to 38%. The fraction of patients in stages 1-3 who never received chemotherapy (neither for frontline nor at recurrence) increased from 35 to 60%. The proportion of macroscopically complete surgical resections of the primary tumor decreased for the total population as well as for patients with stage 4 aged ≥18 months. The impact of chemotherapy response on the outcome was trial dependent. The overall proportion of toxic death during the time of the protocol therapy was 6% for stage 4 patients aged ≥18 months and 2% for low-/intermediate-risk patients. The most frequently reported late sequelae in stage 4 patients aged ≥18 months were renal dysfunctions, hypothyroidism, major hearing impairment, and second malignancies.
Conclusion: The body of data for incidences, risk profiles, and survival rates from this survey of more than 37 years provides a useful perspective for future studies on neuroblastoma sub-cohorts.
Conflict of interest statement
Funding
The clinical trials were funded by the German Ministry of Research and Technology (Grant DLR 01ZP8602/3), the German Children’s Cancer Aid (Deutsche Krebshilfe; Grants 70-443, 70-2290-Be I, T12/97/Be1, 70107712), the German Children’s Cancer Foundation (Deutsche Kinderkrebsstiftung; Grants DKS 95.09; 2004.08; 2006.10), and the Children’s Cancer Research Fund Neuroblastoma (Fördergesellschaft Kinderkrebs-Neuroblastom-Forschung; 3610062131).
Conflict of interest
FB, CS, PK, and FL have no conflicts of interest.
Figures
References
-
- Kaatsch P, Grabow D, Spix C. German Childhood Cancer Registry—Annual Report 2016 (1980–2015). Institute of Medical Biostatistics, Epidemiology and Informatics (IMBEI) at the University Medical Center of the Johannes Gutenberg University Mainz, 2016. http://www.kinderkrebsregister.de. Accessed 20 June 2014.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

