Basolateral amygdala and stress-induced hyperexcitability affect motivated behaviors and addiction
- PMID: 28786979
- PMCID: PMC5611728
- DOI: 10.1038/tp.2017.161
Basolateral amygdala and stress-induced hyperexcitability affect motivated behaviors and addiction
Abstract
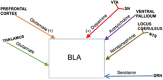
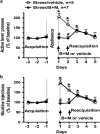
The amygdala integrates and processes incoming information pertinent to reward and to emotions such as fear and anxiety that promote survival by warning of potential danger. Basolateral amygdala (BLA) communicates bi-directionally with brain regions affecting cognition, motivation and stress responses including prefrontal cortex, hippocampus, nucleus accumbens and hindbrain regions that trigger norepinephrine-mediated stress responses. Disruption of intrinsic amygdala and BLA regulatory neurocircuits is often caused by dysfunctional neuroplasticity frequently due to molecular alterations in local GABAergic circuits and principal glutamatergic output neurons. Changes in local regulation of BLA excitability underlie behavioral disturbances characteristic of disorders including post-traumatic stress syndrome (PTSD), autism, attention-deficit hyperactivity disorder (ADHD) and stress-induced relapse to drug use. In this Review, we discuss molecular mechanisms and neural circuits that regulate physiological and stress-induced dysfunction of BLA/amygdala and its principal output neurons. We consider effects of stress on motivated behaviors that depend on BLA; these include drug taking and drug seeking, with emphasis on nicotine-dependent behaviors. Throughout, we take a translational approach by integrating decades of addiction research on animal models and human trials. We show that changes in BLA function identified in animal addiction models illuminate human brain imaging and behavioral studies by more precisely delineating BLA mechanisms. In summary, BLA is required to promote responding for natural reward and respond to second-order drug-conditioned cues; reinstate cue-dependent drug seeking; express stress-enhanced reacquisition of nicotine intake; and drive anxiety and fear. Converging evidence indicates that chronic stress causes BLA principal output neurons to become hyperexcitable.
Conflict of interest statement
The author declares no conflict of interest.
Figures


Similar articles
-
Basolateral amygdala, nicotinic cholinergic receptors, and nicotine: Pharmacological effects and addiction in animal models and humans.Eur J Neurosci. 2019 Aug;50(3):2247-2254. doi: 10.1111/ejn.13970. Epub 2018 Aug 31. Eur J Neurosci. 2019. PMID: 29802666 Review.
-
Cocaine and chronic stress exposure produce an additive increase in neuronal activity in the basolateral amygdala.Addict Biol. 2021 Jan;26(1):e12848. doi: 10.1111/adb.12848. Epub 2019 Nov 21. Addict Biol. 2021. PMID: 31750602 Free PMC article.
-
Increased Basolateral Amygdala Pyramidal Cell Excitability May Contribute to the Anxiogenic Phenotype Induced by Chronic Early-Life Stress.J Neurosci. 2015 Jul 1;35(26):9730-40. doi: 10.1523/JNEUROSCI.0384-15.2015. J Neurosci. 2015. PMID: 26134655 Free PMC article.
-
Glutamatergic mechanisms associated with stress-induced amygdala excitability and anxiety-related behavior.Neuropharmacology. 2014 Oct;85:190-7. doi: 10.1016/j.neuropharm.2014.04.015. Epub 2014 May 4. Neuropharmacology. 2014. PMID: 24796255 Free PMC article.
-
Addiction and brain reward and antireward pathways.Adv Psychosom Med. 2011;30:22-60. doi: 10.1159/000324065. Epub 2011 Apr 19. Adv Psychosom Med. 2011. PMID: 21508625 Free PMC article. Review.
Cited by
-
Genomic glucocorticoid receptor effects guide acute stress-induced delayed anxiety and basolateral amygdala spine plasticity in rats.Neurobiol Stress. 2023 Nov 10;28:100587. doi: 10.1016/j.ynstr.2023.100587. eCollection 2024 Jan. Neurobiol Stress. 2023. PMID: 38075022 Free PMC article.
-
"Preaddiction" construct and reward deficiency syndrome: genetic link via dopaminergic dysregulation.Ann Transl Med. 2022 Nov;10(21):1181. doi: 10.21037/atm-2022-32. Ann Transl Med. 2022. PMID: 36467361 Free PMC article. No abstract available.
-
Role of microRNA-132 in Opioid Addiction through Modification of Neural Stem Cell Differentiation.J Pers Med. 2022 Nov 1;12(11):1800. doi: 10.3390/jpm12111800. J Pers Med. 2022. PMID: 36579528 Free PMC article.
-
α7 nicotinic receptor full agonist reverse basolateral amygdala hyperactivity and attenuation of dopaminergic neuron activity in rats exposed to chronic mild stress.Eur Neuropsychopharmacol. 2019 Dec;29(12):1343-1353. doi: 10.1016/j.euroneuro.2019.09.009. Epub 2019 Oct 12. Eur Neuropsychopharmacol. 2019. PMID: 31615702 Free PMC article.
-
Brain-Based Biotypes of Psychiatric Vulnerability in the Acute Aftermath of Trauma.Am J Psychiatry. 2021 Nov;178(11):1037-1049. doi: 10.1176/appi.ajp.2021.20101526. Epub 2021 Oct 14. Am J Psychiatry. 2021. PMID: 34645277 Free PMC article.
References
-
- Yu X, Liu L, Chen W, Cao Q, Zepf FD, Ji G et al. Integrity of amygdala subregion-based functional networks and emotional lability in drug-naive boys with ADHD. J Atten Disord 2016; 18: pii: pyv0171087054716661419, [e-pub ahead of print]. - PubMed
-
- Capogna M. GABAergic cell type diversity in the basolateral amygdala. Curr Opin Neurobiol 2014; 26: 110–116. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

