Effect of Azithromycin on Airflow Decline-Free Survival After Allogeneic Hematopoietic Stem Cell Transplant: The ALLOZITHRO Randomized Clinical Trial
- PMID: 28787506
- PMCID: PMC5817485
- DOI: 10.1001/jama.2017.9938
Effect of Azithromycin on Airflow Decline-Free Survival After Allogeneic Hematopoietic Stem Cell Transplant: The ALLOZITHRO Randomized Clinical Trial
Abstract
Importance: Bronchiolitis obliterans syndrome has been associated with increased morbidity and mortality after allogeneic hematopoietic stem cell transplant (HSCT). Previous studies have suggested that azithromycin may reduce the incidence of post-lung transplant bronchiolitis obliterans syndrome.
Objective: To evaluate if the early administration of azithromycin can improve airflow decline-free survival after allogeneic HSCT.
Design, setting, and participants: The ALLOZITHRO parallel-group trial conducted in 19 French academic transplant centers and involving participants who were at least 16 years old, had undergone allogeneic HSCT for a hematological malignancy, and had available pretransplant pulmonary function test results. Enrollment was from February 2014 to August 2015 with follow-up through April 26, 2017.
Interventions: Patients were randomly assigned to receive 3 times a week either 250 mg of azithromycin (n = 243) or placebo (n = 237) for 2 years, starting at the time of the conditioning regimen.
Main outcomes and measures: The primary efficacy end point was airflow decline-free survival at 2 years after randomization. Main secondary end points were overall survival and bronchiolitis obliterans syndrome at 2 years.
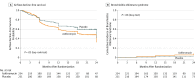
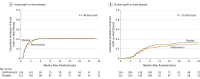
Results: Thirteen months after enrollment, the independent data and safety monitoring board detected an unanticipated imbalance across blinded groups in the number of hematological relapses, and the treatment was stopped December 26, 2016. Among 480 randomized participants, 465 (97%) were included in the modified intention-to-treat analysis (mean age, 52 [SD, 14] years; 75 women [35%]). At the time of data cutoff, 104 patients (22%; 54 azithromycin vs 50 placebo) had experienced an airflow decline; 138 patients (30%) died (78 azithromycin vs 60 placebo). Two-year airflow decline-free survival was 32.8% (95% CI, 25.9%-41.7%) with azithromycin and 41.3% (95% CI, 34.1%-50.1%) with placebo (unadjusted hazard ratio [HR], 1.3; 95% CI, 1.02-1.70; P = .03). Of the 22 patients (5%) who experienced bronchiolitis obliterans syndrome, 15 (6%) were in the azithromycin group and 7 (3%) in the placebo group (P = .08). The azithromycin group had increased mortality, with a 2-year survival of 56.6% (95% CI, 50.2%-63.7%) vs 70.1% (95% CI, 64.2%-76.5%) in the placebo group (unadjusted HR, 1.5; 95% CI, 1.1-2.0; P = .02). In a post hoc analysis, the 2-year cumulative incidence of hematological relapse was 33.5% (95% CI, 27.3%-39.7%) with azithromycin vs 22.3% (95% CI, 16.4%-28.2%) with placebo (unadjusted cause-specific HR, 1.7; 95% CI, 1.2-2.4; P = .002).
Conclusions and relevance: Among patients undergoing allogeneic HSCT for hematological malignancy, early administration of azithromycin resulted in worse airflow decline-free survival than did placebo; these findings are limited by early trial termination. The potential for harm related to relapse requires further investigation.
Trial registration: clinicaltrials.gov Identifier: NCT01959100.
Conflict of interest statement
Figures


Comment in
-
Azithromycin and Survival After Hematopoietic Stem Cell Transplant.JAMA. 2017 Dec 26;318(24):2492. doi: 10.1001/jama.2017.17228. JAMA. 2017. PMID: 29279920 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

