The Helicase Aquarius/EMB-4 Is Required to Overcome Intronic Barriers to Allow Nuclear RNAi Pathways to Heritably Silence Transcription
- PMID: 28787591
- PMCID: PMC5554785
- DOI: 10.1016/j.devcel.2017.07.002
The Helicase Aquarius/EMB-4 Is Required to Overcome Intronic Barriers to Allow Nuclear RNAi Pathways to Heritably Silence Transcription
Abstract
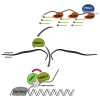
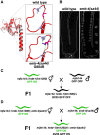
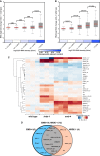
Small RNAs play a crucial role in genome defense against transposable elements and guide Argonaute proteins to nascent RNA transcripts to induce co-transcriptional gene silencing. However, the molecular basis of this process remains unknown. Here, we identify the conserved RNA helicase Aquarius/EMB-4 as a direct and essential link between small RNA pathways and the transcriptional machinery in Caenorhabditis elegans. Aquarius physically interacts with the germline Argonaute HRDE-1. Aquarius is required to initiate small-RNA-induced heritable gene silencing. HRDE-1 and Aquarius silence overlapping sets of genes and transposable elements. Surprisingly, removal of introns from a target gene abolishes the requirement for Aquarius, but not HRDE-1, for small RNA-dependent gene silencing. We conclude that Aquarius allows small RNA pathways to compete for access to nascent transcripts undergoing co-transcriptional splicing in order to detect and silence transposable elements. Thus, Aquarius and HRDE-1 act as gatekeepers coordinating gene expression and genome defense.
Keywords: C. elegans; Piwi; RNA processing; RNAi; epigenetic inheritance; nuclear RNAi; piRNA; splicing; transcription; transposable elements.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- de Albuquerque B.F.M., Placentino M., Ketting R.F. Maternal piRNAs are essential for germline development following de novo establishment of endo-siRNAs in Caenorhabditis elegans. Dev. Cell. 2015;34:448–456. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

