Persistent Pulmonary Hypertension in the Newborn
- PMID: 28788074
- PMCID: PMC5575585
- DOI: 10.3390/children4080063
Persistent Pulmonary Hypertension in the Newborn
Abstract
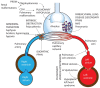
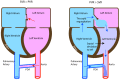
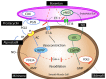
Persistent pulmonary hypertension of the newborn (PPHN) is a syndrome of failed circulatory adaptation at birth due to delay or impairment in the normal fall in pulmonary vascular resistance (PVR) that occurs following birth. The fetus is in a state of physiological pulmonary hypertension. In utero, the fetus receives oxygenated blood from the placenta through the umbilical vein. At birth, following initiation of respiration, there is a sudden precipitous fall in the PVR and an increase of systemic vascular resistance (SVR) due to the removal of the placenta from circulation. There is dramatic increase in pulmonary blood flow with a decrease in, and later reversal of shunts at the foramen ovale and ductus arteriosus. The failure of this normal physiological pulmonary transition leads to the syndrome of PPHN. PPHN presents with varying degrees of hypoxemic respiratory failure. Survival of infants with PPHN has significantly improved with the use of gentle ventilation, surfactant and inhaled nitric oxide (iNO). PPHN is associated with significant mortality and morbidity among survivors. Newer agents that target different enzymatic pathways in the vascular smooth muscle are in different stages of development and testing. Further research using these agents is likely to further reduce morbidity and mortality associated with PPHN.
Keywords: hypoxemia; nitric oxide; oxygen; pulmonary blood flow.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Lakshminrusimha S., Steinhorn R.H. Pulmonary vascular biology during neonatal transition. Clin. Perinatol. 1999;26:601–619. - PubMed
-
- Walsh-Sukys M.C., Tyson J.E., Wright L.L., Bauer C.R., Korones S.B., Stevenson D.K., Verter J., Stoll B.J., Lemons J.A., Papile L.A., et al. Persistent pulmonary hypertension of the newborn in the era before nitric oxide: Practice variation and outcomes. Pediatrics. 2000;105:14–20. doi: 10.1542/peds.105.1.14. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

