Cellular Reparative Mechanisms of Mesenchymal Stem Cells for Retinal Diseases
- PMID: 28788088
- PMCID: PMC5577990
- DOI: 10.3390/ijms18081406
Cellular Reparative Mechanisms of Mesenchymal Stem Cells for Retinal Diseases
Abstract
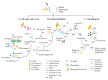
The use of multipotent mesenchymal stem cells (MSCs) has been reported as promising for the treatment of numerous degenerative disorders including the eye. In retinal degenerative diseases, MSCs exhibit the potential to regenerate into retinal neurons and retinal pigmented epithelial cells in both in vitro and in vivo studies. Delivery of MSCs was found to improve retinal morphology and function and delay retinal degeneration. In this review, we revisit the therapeutic role of MSCs in the diseased eye. Furthermore, we reveal the possible cellular mechanisms and identify the associated signaling pathways of MSCs in reversing the pathological conditions of various ocular disorders such as age-related macular degeneration (AMD), retinitis pigmentosa, diabetic retinopathy, and glaucoma. Current stem cell treatment can be dispensed as an independent cell treatment format or with the combination of other approaches. Hence, the improvement of the treatment strategy is largely subjected by our understanding of MSCs mechanism of action.
Keywords: MSC differentiation; anti-angiogenesis; anti-inflammatory; immunomodulatory; mesenchymal stem cells; paracrine activity; retinal degenerative diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Triviño A., de Hoz R., Rojas B., Gallego B.I., Ramírez A.I., Salazar J.J., Ramírez J.M. Ocular Diseases. InTech; Rijeka, Croatia: 2012. Effects of Hypercholesterolaemia in the Retina.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

