Alginate-Poly(ethylene glycol) Hybrid Microspheres for Primary Cell Microencapsulation
- PMID: 28788456
- PMCID: PMC5453158
- DOI: 10.3390/ma7010275
Alginate-Poly(ethylene glycol) Hybrid Microspheres for Primary Cell Microencapsulation
Abstract
The progress of medical therapies, which rely on the transplantation of microencapsulated living cells, depends on the quality of the encapsulating material. Such material has to be biocompatible, and the microencapsulation process must be simple and not harm the cells. Alginate-poly(ethylene glycol) hybrid microspheres (alg-PEG-M) were produced by combining ionotropic gelation of sodium alginate (Na-alg) using calcium ions with covalent crosslinking of vinyl sulfone-terminated multi-arm poly(ethylene glycol) (PEG-VS). In a one-step microsphere formation process, fast ionotropic gelation yields spherical calcium alginate gel beads, which serve as a matrix for simultaneously but slowly occurring covalent cross-linking of the PEG-VS molecules. The feasibility of cell microencapsulation was studied using primary human foreskin fibroblasts (EDX cells) as a model. The use of cell culture media as polymer solvent, gelation bath, and storage medium did not negatively affect the alg-PEG-M properties. Microencapsulated EDX cells maintained their viability and proliferated. This study demonstrates the feasibility of primary cell microencapsulation within the novel microsphere type alg-PEG-M, serves as reference for future therapy development, and confirms the suitability of EDX cells as control model.
Keywords: alginate; biocompatibility; cell encapsulation; cell transplantation; hydrogel; microencapsulation; poly(ethylene glycol).
Conflict of interest statement
The authors declare no conflict of interest.
Figures


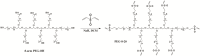
References
-
- Bonavita A.G., Quaresma K., Cotta-de-Almeida V., Pinto M.A., Saraiva R.M., Alves L.A. Hepatocyte xenotransplantation for treating liver disease. Xenotransplantation. 2010;17:181–187. - PubMed
-
- Paul A., Ge Y., Prakash S., Shum-Tim D. Microencapsulated stem cells for tissue repairing: Implications in cell-based myocardial therapy. Regener. Med. 2009;4:733–745. - PubMed
-
- De Vos P., Faas M.M., Strand B., Calafiore R. Alginate-based microcapsules for immunoisolation of pancreatic islets. Biomaterials. 2006;27:5603–5617. - PubMed
-
- Hernández R.M., Orive G., Murara A., Pedraz J.L. Microcapsules and microcarriers for in situ cell delivery. Adv. Drug Deliv. Rev. 2010;62:711–730. - PubMed
-
- Rokstad A.M., Brekke O.L., Steinkjer B., Ryan L., Kolláriková G., Strand B.L., Skjåk-Braek G., Lacik I., Espevik T., Mollnes T.E. Alginate microbeads are complement compatible, in contrast to polycation containing microcapsules, as revealed in a human whole blood model. Acta Biomater. 2011;7(1):2566–2578. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

