Wear Debris Characterization and Corresponding Biological Response: Artificial Hip and Knee Joints
- PMID: 28788496
- PMCID: PMC5453097
- DOI: 10.3390/ma7020980
Wear Debris Characterization and Corresponding Biological Response: Artificial Hip and Knee Joints
Abstract
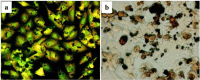
Wear debris, of deferent sizes, shapes and quantities, generated in artificial hip and knees is largely confined to the bone and joint interface. This debris interacts with periprosthetic tissue and may cause aseptic loosening. The purpose of this review is to summarize and collate findings of the recent demonstrations on debris characterization and their biological response that influences the occurrence in implant migration. A systematic review of peer-reviewed literature is performed, based on inclusion and exclusion criteria addressing mainly debris isolation, characterization, and biologic responses. Results show that debris characterization largely depends on their appropriate and accurate isolation protocol. The particles are found to be non-uniform in size and non-homogeneously distributed into the periprosthetic tissues. In addition, the sizes, shapes, and volumes of the particles are influenced by the types of joints, bearing geometry, material combination, and lubricant. Phagocytosis of wear debris is size dependent; high doses of submicron-sized particles induce significant level of secretion of bone resorbing factors. However, articles on wear debris from engineered surfaces (patterned and coated) are lacking. The findings suggest considering debris morphology as an important parameter to evaluate joint simulator and newly developed implant materials.
Keywords: biological response; isolation; morphology; nano-toxicity; wear debris.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Kurtz S., Ong K., Lau E., Mowat F., Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Joint Surg. Am. 2007;89:780–785. - PubMed
-
- Harris W.H. The problem is osteolysis. Clin. Orthop. Relat. Res. 1995;311:46–53. - PubMed
-
- Callaghan J.J., O’rourke M.R., Saleh K.J. Why knees fail: Lessons learned. J. Arthroplast. 2004;19:31–34. - PubMed
-
- Ingham E., Fisher J. The role of macrophages in osteolysis of total joint replacement. Biomaterials. 2005;26:1271–1286. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

