Detection of Waterborne and Airborne Formaldehyde: From Amperometric Chemosensing to a Visual Biosensor Based on Alcohol Oxidase
- PMID: 28788499
- PMCID: PMC5453092
- DOI: 10.3390/ma7021055
Detection of Waterborne and Airborne Formaldehyde: From Amperometric Chemosensing to a Visual Biosensor Based on Alcohol Oxidase
Abstract
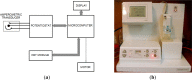
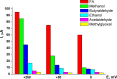
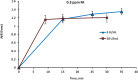
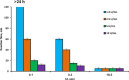
A laboratory prototype of a microcomputer-based analyzer was developed for quantitative determination of formaldehyde in liquid samples, based on catalytic chemosensing elements. It was shown that selectivity for the target analyte could be increased by modulating the working electrode potential. Analytical parameters of three variants of the amperometric analyzer that differed in the chemical structure/configuration of the working electrode were studied. The constructed analyzer was tested on wastewater solutions that contained formaldehyde. A simple low-cost biosensor was developed for semi-quantitative detection of airborne formaldehyde in concentrations exceeding the threshold level. This biosensor is based on a change in the color of a solution that contains a mixture of alcohol oxidase from the yeast Hansenula polymorpha, horseradish peroxidase and a chromogen, following exposure to airborne formaldehyde. The solution is enclosed within a membrane device, which is permeable to formaldehyde vapors. The most efficient and sensitive biosensor for detecting formaldehyde was the one that contained alcohol oxidase with an activity of 1.2 U·mL-1. The biosensor requires no special instrumentation and enables rapid visual detection of airborne formaldehyde at concentrations, which are hazardous to human health.
Keywords: Hansenula polymorpha; biosensor, alcohol oxidase; chemosensing electrode; formaldehyde; microcomputer-based analyzer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Cogliano V.J., Grosse Y., Baan R.A., Straif K., Secretan M.B., El Ghissassi F. Formaldehyde, 2-Butoxyethanol, and 1-tert-Butoxy-2-Propanol. IARC Monographs on the Evaluation of Carcinogenic Risks to Human, IARC Monographs. Vol. 88. WHO Press; Lyon, France: 2006. Formaldehyde; pp. 39–93.
-
- Blair A., Casanova M., Comba P., Demers P., Goldstein B.D., Grafström R.C., Järvholm B., Kaufman D.G., Kauppinen T., Leclerc A., et al. Wood Dust and Formaldehyde. IARC Monographs on Evaluation of Carcinogenic Risks to Humans. Vol. 62. WHO Press; Lyon, France: 1995. Formaldehyde; pp. 217–335.
-
- Barrett J.C., Bond J.A., Beane-Freeman L., Carren-Valencia T., Elwell M.R., Groopman J.D., Friesen M.D., Gustavsson P., Ghanei M., Hayes R.B., et al. Chemical Agentsand Related Occupations. 100F. WHO Press; Lyon, France; 2012. Formaldehyde; pp. 401–435.
-
- Occupational Exposure Limits for Formaldehyde. [(accessed on 1 July 2009)]. Available online: http://www.worksafebc.com/regulation_and_policy/policy_decision/board_de....
-
- Feron V.J., Til H.P., Vrijer F., Woutersen R.A., Cassee F.R., van Bladeren P.J. Aldehydes: Occurrence, carcinogenic potential, mechanism of action and risk assessment. Mutat. Res. 1991;259:363–385. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

