Role and mechanism of decitabine combined with tyrosine kinase inhibitors in advanced chronic myeloid leukemia cells
- PMID: 28789344
- PMCID: PMC5529866
- DOI: 10.3892/ol.2017.6318
Role and mechanism of decitabine combined with tyrosine kinase inhibitors in advanced chronic myeloid leukemia cells
Abstract
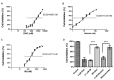
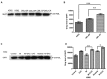
Patients with advanced chronic myeloid leukemia (CML) have a poor prognosis, with the use of tyrosine kinase inhibitors (TKIs) to treat CML demonstrating poor results. The results of the present study revealed that, following Cell Counting Kit-8 analysis, treatment of K562 cells with decitabine (DAC) combined with TKIs exhibits synergic effects. Co-immunoprecipitation indicated that tyrosine-protein phosphatase non-receptor type 6 (SHP-1) and BCR-ABL fusion protein (BCR-ABL) (p210) form a complex in the K562 cell line, and in the primary cells derived from patients with CML. These results suggested that SHP-1 serves a role in regulating the tyrosine kinase activity of BCR-ABL (p210). In addition, SHP-1 expression increased, while BCR-ABL expression decreased in the group treated with DAC and TKIs combined group compared with the TKI monotherapy group. Treatment with imatinib (IM) demonstrated no effect on SHP-1 methylation in the K562 cell line; however, the methylation of SHP-1 was not determined in the combined IM and DAC therapy group. Treatment with DAC demonstrated the ability to activate the expression of silenced SHP-1 through demethylation, thus decreasing BCR-ABL tyrosine kinase activity, resulting in an improved therapeutic effect on CML.
Keywords: chronic myeloid leukemia; demethylation; immunoprecipitation; tyrosine kinase inhibitors; tyrosine-protein phosphatase non-receptor type 6.
Figures



Similar articles
-
[Overexpression of Shp-2 is associated with the unlimited growth and apoptosis resistance of p210 bcr-abl-mediated chronic myeloid leukemia].Zhonghua Yi Xue Za Zhi. 2005 Jul 20;85(27):1903-6. Zhonghua Yi Xue Za Zhi. 2005. PMID: 16255985 Chinese.
-
[SHP-1 gene in the disease progression of chronic myeloid leukemia].Zhonghua Xue Ye Xue Za Zhi. 2014 Dec;35(12):1074-8. doi: 10.3760/cma.j.issn.0253-2727.2014.12.006. Zhonghua Xue Ye Xue Za Zhi. 2014. PMID: 25543700 Chinese.
-
Bone marrow-derived mesenchymal stromal cells promote resistance to tyrosine kinase inhibitors in chronic myeloid leukemia via the IL-7/JAK1/STAT5 pathway.J Biol Chem. 2019 Aug 9;294(32):12167-12179. doi: 10.1074/jbc.RA119.008037. Epub 2019 Jun 24. J Biol Chem. 2019. PMID: 31235520 Free PMC article.
-
BCR-ABL tyrosine kinase inhibitors in the treatment of Philadelphia chromosome positive chronic myeloid leukemia: a review.Leuk Res. 2010 Oct;34(10):1255-68. doi: 10.1016/j.leukres.2010.04.016. Leuk Res. 2010. PMID: 20537386 Review.
-
[MET/ERK and MET/JNK Pathway Activation Is Involved in BCR-ABL Inhibitor-resistance in Chronic Myeloid Leukemia].Yakugaku Zasshi. 2018;138(12):1461-1466. doi: 10.1248/yakushi.18-00142. Yakugaku Zasshi. 2018. PMID: 30504658 Review. Japanese.
Cited by
-
Phase I/II study of dasatinib in combination with decitabine in patients with accelerated or blast phase chronic myeloid leukemia.Am J Hematol. 2020 Nov;95(11):1288-1295. doi: 10.1002/ajh.25939. Epub 2020 Aug 12. Am J Hematol. 2020. PMID: 32681739 Free PMC article. Clinical Trial.
-
Deletion of MBD2 inhibits proliferation of chronic myeloid leukaemia blast phase cells.Cancer Biol Ther. 2018 Aug 3;19(8):676-686. doi: 10.1080/15384047.2018.1450113. Epub 2018 Apr 19. Cancer Biol Ther. 2018. PMID: 29565710 Free PMC article.
-
Cancer Treatment: An Epigenetic View.Glob Med Genet. 2020 Jun;7(1):3-7. doi: 10.1055/s-0040-1713610. Epub 2020 Jul 15. Glob Med Genet. 2020. PMID: 32879917 Free PMC article. Review.
-
Decitabine Downregulates TIGAR to Induce Apoptosis and Autophagy in Myeloid Leukemia Cells.Oxid Med Cell Longev. 2021 Jan 18;2021:8877460. doi: 10.1155/2021/8877460. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 33532040 Free PMC article.
-
HNRNPH1 Is a Novel Regulator Of Cellular Proliferation and Disease Progression in Chronic Myeloid Leukemia.Front Oncol. 2021 Jul 6;11:682859. doi: 10.3389/fonc.2021.682859. eCollection 2021. Front Oncol. 2021. PMID: 34295818 Free PMC article.
References
-
- National Comprehensive Cancer Network, corp-author. NCCN Clinical Practice Guidelines in Oncology: Chronic Myelogenous Leukemia, V.2.[DB/OL].(2007-10-25) 2008 - PubMed
-
- Druker BJ, Lee SJ. Chronic myelogenous leukemia. Cancer: Principles and Practice of Oncology. 2007
-
- Incidence survey of leukemia in China, corp-author. Chinese Epidemiologic Study Group of Leukemia and Aplastic Anemia. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 1992;14:12–19. (In Chinese) - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
