Analysis of health-related quality of life in patients with brain tumors prior and subsequent to radiotherapy
- PMID: 28789419
- PMCID: PMC5529906
- DOI: 10.3892/ol.2017.6310
Analysis of health-related quality of life in patients with brain tumors prior and subsequent to radiotherapy
Abstract
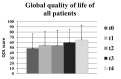
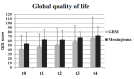
Health-related quality of life (HRQOL) was evaluated for a patient cohort with benign or malignant brain tumors prior and subsequent to radiotherapy. The following inclusion criteria were applied: Sufficient compliance, understanding of patient information and the existence of a brain tumor without previous radiotherapy. Patients were asked to complete the European Organization for Research and Treatment of Cancer QLQ-C30 and QLQ-BN20 questionnaires at the following times: Prior to (t0) and subsequent to (t1) radiotherapy, and at 3 (t2), 6 (t3) and 12 (t4) months following treatment. In addition, at t1 the side effects were assessed according to the Common Terminology Criteria for Adverse Events. Generally, the global QOL, a standard term describing general QOL, improved slightly (t0=49; t4=65). At t1, a significant increase in fatigue, loss of appetite and alopecia was reported. During follow-up, the symptoms experienced by the patients decreased, and the global QOL remained constant. The objectively recorded side effects of the therapy were comparable with the patient-reported outcomes.
Keywords: brain tumor; glioblastoma; meningioma; quality of life.
Figures




References
-
- Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. - DOI - PubMed
-
- Velikova G, Coens C, Efficace F, Greimel E, Groenvold M, Johnson C, Singer S, van de Poll-Franse L, Young T, Bottomley A. Health-related quality of life in EORTC clinical trials-30 years of progress from methodological developments to making a real impact on oncology practice. EJC Suppl. 2012;10:141–149. doi: 10.1016/S1359-6349(12)70023-X. - DOI
-
- Steinmann D, Vordermark D, Geinitz H, Aschoff R, Bayerl A, Gerstein J, Hipp M, van Oorschot B, Wypior HJ, Schäfer C. Proxy assessment of patients before and after radiotherapy for brain metastases. Results of a prospective study using the DEGRO brain module. Strahlenther Onkol. 2013;189:47–53. doi: 10.1007/s00066-012-0239-4. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
