Adiponectin protects against development of metabolic disturbances in a PCOS mouse model
- PMID: 28790184
- PMCID: PMC5576831
- DOI: 10.1073/pnas.1708854114
Adiponectin protects against development of metabolic disturbances in a PCOS mouse model
Abstract
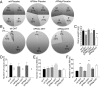
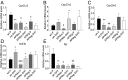
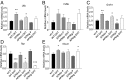
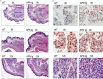
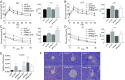
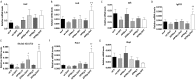
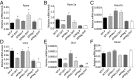
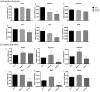
Adiponectin, together with adipocyte size, is the strongest factor associated with insulin resistance in women with polycystic ovary syndrome (PCOS). This study investigates the causal relationship between adiponectin levels and metabolic and reproductive functions in PCOS. Prepubertal mice overexpressing adiponectin from adipose tissue (APNtg), adiponectin knockouts (APNko), and their wild-type (WT) littermate mice were continuously exposed to placebo or dihydrotestosterone (DHT) to induce PCOS-like traits. As expected, DHT exposure led to reproductive dysfunction, as judged by continuous anestrus, smaller ovaries with a decreased number of corpus luteum, and an increased number of cystic/atretic follicles. A two-way between-groups analysis showed that there was a significant main effect for DHT exposure, but not for genotype, indicating adiponectin does not influence follicle development. Adiponectin had, however, some protective effects on ovarian function. Similar to in many women with PCOS, DHT exposure led to reduced adiponectin levels, larger adipocyte size, and reduced insulin sensitivity in WTs. APNtg mice remained metabolically healthy despite DHT exposure, while APNko-DHT mice were even more insulin resistant than their DHT-exposed littermate WTs. DHT exposure also reduced the mRNA expression of genes involved in metabolic pathways in gonadal adipose tissue of WT and APNko, but this effect of DHT was not observed in APNtg mice. Moreover, APNtg-DHT mice displayed increased pancreatic mRNA levels of insulin receptors, Pdx1 and Igf1R, suggesting adiponectin stimulates beta cell viability/hyperplasia in the context of PCOS. In conclusion, adiponectin improves metabolic health but has only minor effects on reproductive functions in this PCOS-like mouse model.
Keywords: adipose tissue; insulin resistance; polycystic ovary syndrome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Corbould A. Insulin resistance in skeletal muscle and adipose tissue in polycystic ovary syndrome: Are the molecular mechanisms distinct from type 2 diabetes? Panminerva Med. 2008;50:279–294. - PubMed
-
- Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: A prospective, controlled study in 254 affected women. J Clin Endocrinol Metab. 1999;84:165–169. - PubMed
-
- Ehrmann DA, Barnes RB, Rosenfield RL, Cavaghan MK, Imperial J. Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care. 1999;22:141–146. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

