Unraveling heme detoxification in the malaria parasite by in situ correlative X-ray fluorescence microscopy and soft X-ray tomography
- PMID: 28790371
- PMCID: PMC5548722
- DOI: 10.1038/s41598-017-06650-w
Unraveling heme detoxification in the malaria parasite by in situ correlative X-ray fluorescence microscopy and soft X-ray tomography
Abstract
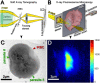
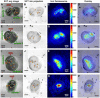
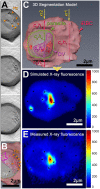
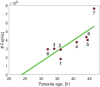
A key drug target for malaria has been the detoxification pathway of the iron-containing molecule heme, which is the toxic byproduct of hemoglobin digestion. The cornerstone of heme detoxification is its sequestration into hemozoin crystals, but how this occurs remains uncertain. We report new results of in vivo rate of heme crystallization in the malaria parasite, based on a new technique to measure element-specific concentrations at defined locations in cell ultrastructure. Specifically, a high resolution correlative combination of cryo soft X-ray tomography has been developed to obtain 3D parasite ultrastructure with cryo X-ray fluorescence microscopy to measure heme concentrations. Our results are consistent with a model for crystallization via the heme detoxification protein. Our measurements also demonstrate the presence of considerable amounts of non-crystalline heme in the digestive vacuole, which we show is most likely contained in hemoglobin. These results suggest a tight coupling between hemoglobin digestion and heme crystallization, highlighting a new link in the crystallization pathway for drug development.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

