Mast Cells and IgE can Enhance Survival During Innate and Acquired Host Responses to Venoms
- PMID: 28790503
- PMCID: PMC5525434
Mast Cells and IgE can Enhance Survival During Innate and Acquired Host Responses to Venoms
Abstract
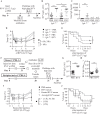
Mast cells and immunoglobulin E (IgE) antibodies are thought to promote health by contributing to host responses to certain parasites, but other beneficial functions have remained obscure. Venoms provoke innate inflammatory responses and pathology reflecting the activities of the contained toxins. Venoms also can induce allergic sensitization and development of venom-specific IgE antibodies, which can predispose some subjects to exhibit anaphylaxis upon subsequent exposure to the relevant venom. We found that innate functions of mast cells, including degradation of venom toxins by mast cell-derived proteases, enhanced survival in mice injected with venoms from the honeybee, two species of scorpion, three species of poisonous snakes, or the Gila monster. We also found that mice injected with sub-lethal amounts of honeybee or Russell's viper venom exhibited enhanced survival after subsequent challenge with potentially lethal amounts of that venom, and that IgE antibodies, FcεRI, and probably mast cells contributed to such acquired resistance.
Conflict of interest statement
Potential Conflicts of Interest: The work reviewed herein was supported by grants to Dr. Galli from the National Institutes of Health (e.g., R37 AI23990, R01 CA072074, R01 AR067145, and U19 AI104209) and the National Science Foundation, and from several other funding sources, including the Department of Pathology at Stanford University. Dr. Starkl was supported by a Max Kade Fellowship of the Max Kade Foundation and the Austrian Academy of Sciences, a Schroedinger Fellowship of the Austrian Science Fund (FWF): J3399-B21, and a Marie Curie fellowship of the European Commission (H2020-MSCA-IF-2014), 655153. Dr. Marichal was supported by a Marie Curie International Outgoing Fellowship for Career Development: European Union’s Seventh Framework Programme (FP7-PEOPLE-2011-IOF), 299954, and a “Charge de recherches” fellowship of the Belgian National Fund for Scientific Research (F.R.S-FNRS).
Figures




Similar articles
-
Testing the 'toxin hypothesis of allergy': mast cells, IgE, and innate and acquired immune responses to venoms.Curr Opin Immunol. 2015 Oct;36:80-7. doi: 10.1016/j.coi.2015.07.001. Epub 2015 Jul 23. Curr Opin Immunol. 2015. PMID: 26210895 Free PMC article. Review.
-
Mast cells and IgE in defense against venoms: Possible "good side" of allergy?Allergol Int. 2016 Jan;65(1):3-15. doi: 10.1016/j.alit.2015.09.002. Epub 2015 Oct 23. Allergol Int. 2016. PMID: 26666482 Review.
-
The Mast Cell-IgE Paradox: From Homeostasis to Anaphylaxis.Am J Pathol. 2016 Feb;186(2):212-24. doi: 10.1016/j.ajpath.2015.07.025. Am J Pathol. 2016. PMID: 26776074 Free PMC article. Review.
-
Mast cells and IgE in defense against lethality of venoms: Possible "benefit" of allergy[].Allergo J Int. 2020 Mar;29(2):46-62. doi: 10.1007/s40629-020-00118-6. Epub 2020 Mar 2. Allergo J Int. 2020. PMID: 33224714 Free PMC article.
-
IgE antibodies, FcεRIα, and IgE-mediated local anaphylaxis can limit snake venom toxicity.J Allergy Clin Immunol. 2016 Jan;137(1):246-257.e11. doi: 10.1016/j.jaci.2015.08.005. Epub 2015 Sep 26. J Allergy Clin Immunol. 2016. PMID: 26410782 Free PMC article.
Cited by
-
Mast Cells in the Skin: Defenders of Integrity or Offenders in Inflammation?Int J Mol Sci. 2021 Apr 27;22(9):4589. doi: 10.3390/ijms22094589. Int J Mol Sci. 2021. PMID: 33925601 Free PMC article. Review.
-
Amplification of Snake Venom Toxicity by Endogenous Signaling Pathways.Toxins (Basel). 2020 Jan 22;12(2):68. doi: 10.3390/toxins12020068. Toxins (Basel). 2020. PMID: 31979014 Free PMC article. Review.
-
The functional biology of peanut allergens and possible links to their allergenicity.Allergy. 2019 May;74(5):888-898. doi: 10.1111/all.13719. Epub 2019 Feb 1. Allergy. 2019. PMID: 30636003 Free PMC article. Review.
-
The relationship between ovarian hormones and mast cell distribution in the ovaries of dromedary camel (Camelus dromedaries) during the follicular wave.Vet World. 2023 Feb;16(2):309-316. doi: 10.14202/vetworld.2023.309-316. Epub 2023 Feb 17. Vet World. 2023. PMID: 37041993 Free PMC article.
-
Melanotan II causes hypothermia in mice by activation of mast cells and stimulation of histamine 1 receptors.Am J Physiol Endocrinol Metab. 2018 Sep 1;315(3):E357-E366. doi: 10.1152/ajpendo.00024.2018. Epub 2018 May 29. Am J Physiol Endocrinol Metab. 2018. PMID: 29812984 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
