Bilateral implantation of +3.0 D multifocal toric intraocular lenses: results of a US Food and Drug Administration clinical trial
- PMID: 28790805
- PMCID: PMC5530114
- DOI: 10.2147/OPTH.S137413
Bilateral implantation of +3.0 D multifocal toric intraocular lenses: results of a US Food and Drug Administration clinical trial
Abstract
Purpose: The purpose of this study was to evaluate the clinical outcomes of apodized diffractive +3.0 D multifocal toric intraocular lens (IOL) implantations in subjects with preoperative corneal astigmatism.
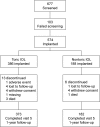
Patients and methods: This was a prospective cohort study conducted at 21 US sites. The study population consisted of 574 subjects, aged ≥21 years, with preoperative astigmatism 0.75-2.82 D, and potential postoperative visual acuity (VA) ≥0.2 logMAR, undergoing bilateral cataract removal by phacoemulsification. The intervention was bilateral implantation of aspheric apodized diffractive +3.0 D multifocal toric or spherical multifocal nontoric IOLs. The main outcome measures were monocular uncorrected near and distance VA and safety at 12 months.
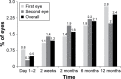
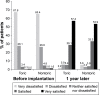
Results: A total of 373/386 and 182/188 subjects implanted with multifocal toric and nontoric IOLs, respectively, completed 12-month follow-up after the second implantation. Toric IOLs were nonin-ferior in monocular uncorrected distance (4 m) and near (40 cm) VA but had >1 line better binocular uncorrected intermediate VA (50, 60, and 70 cm) than nontoric IOLs. Toric IOLs reduced cylinder to within 0.50 D and 1.0 D of target in 278 (74.5%) and 351 (94.1%) subjects, respectively. Mean ± standard deviation (SD) differences between intended and achieved axis orientation in the first and second implanted eyes were 5.0°±6.1° and 4.7°±4.0°, respectively. Mean ± SD 12-month IOL rotations in the first and second implanted eyes were 2.7°±5.8° and 2.2°±2.7°, respectively. No subject receiving toric IOLs required secondary surgical intervention due to optical lens properties.
Conclusion: Multifocal toric IOLs were noninferior to multifocal nontoric IOLs in uncorrected distance and near VAs in subjects with preexisting corneal astigmatism and effectively corrected astigmatism of 0.75-2.82 D.
Keywords: AcrySof® IQ ReSTOR; IOL rotation lens; axis orientation; corneal astigmatism; phacoemulsification; target cylinder; visual acuity.
Conflict of interest statement
Disclosure Robert Lehmann is a consultant to Alcon and has received grant and research support from the sponsor. Satish Modi is a consultant to Alcon and has received grant and research support from the sponsor. Bret Fisher is a consultant to Alcon and has received grant and research support from the sponsor. Magda Michna is an employee of Alcon. Michael Snyder is a consultant to Alcon and has received grant and research support from the sponsor. The authors report no other conflicts of interest in this work.
Figures
References
-
- Ferrer-Blasco T, Montes-Mico R, Peixoto-de-Matos SC, Gonzalez-Meijome JM, Cervino A. Prevalence of corneal astigmatism before cataract surgery. J Cataract Refract Surg. 2009;35(1):70–75. - PubMed
-
- Khan MI, Muhtaseb M. Prevalence of corneal astigmatism in patients having routine cataract surgery at a teaching hospital in the United Kingdom. J Cataract Refract Surg. 2011;37(10):1751–1755. - PubMed
-
- Ernest P, Potvin R. Effects of preoperative corneal astigmatism orientation on results with a low-cylinder-power toric intraocular lens. J Cataract Refract Surg. 2011;37(4):727–732. - PubMed
-
- Statham M, Apel A, Stephensen D. Comparison of the AcrySof SA60 spherical intraocular lens and the AcrySof Toric SN60T3 intraocular lens outcomes in patients with low amounts of corneal astigmatism. Clin Experiment Ophthalmol. 2009;37(8):775–779. - PubMed
-
- Holland E, Lane S, Horn JD, Ernest P, Arleo R, Miller KM. The AcrySof Toric intraocular lens in subjects with cataracts and corneal astigmatism: a randomized, subject-masked, parallel-group, 1-year study. Ophthalmology. 2010;117(11):2104–2111. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

