The Calcineurin-Binding, Activity-Dependent Splice Variant Dynamin1xb Is Highly Enriched in Synapses in Various Regions of the Central Nervous System
- PMID: 28790889
- PMCID: PMC5524891
- DOI: 10.3389/fnmol.2017.00230
The Calcineurin-Binding, Activity-Dependent Splice Variant Dynamin1xb Is Highly Enriched in Synapses in Various Regions of the Central Nervous System
Abstract
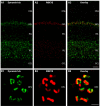
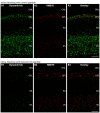
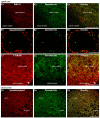
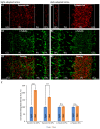
In the present study, we generated and characterized a splice site-specific monoclonal antibody that selectively detects the calcineurin-binding dynamin1 splice variant dynamin1xb. Calcineurin is a Ca2+-regulated phosphatase that enhances dynamin1 activity and is an important Ca2+-sensing mediator of homeostatic synaptic plasticity in neurons. Using this dynamin1xb-specific antibody, we found dynamin1xb highly enriched in synapses of all analyzed brain regions. In photoreceptor ribbon synapses, dynamin1xb was enriched in close vicinity to the synaptic ribbon in a manner indicative of a peri-active zone immunolabeling. Interestingly, in dark-adapted mice we observed an enhanced and selective enrichment of dynamin1xb in both synaptic layers of the retina in comparison to light-adapted mice. This could be due to an illumination-dependent recruitment of dynamin1xb to retinal synapses and/or due to a darkness-induced increase of dynamin1xb biosynthesis. These latter findings indicate that dynamin1xb is part of a versatile and highly adjustable, activity-regulated endocytic synaptic machinery.
Keywords: calcineurin; darkness-induced synaptic recruitment of dynamin1xb; dynamin1xb; retina; splice variant; synapse.
Figures





References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

