Brain and Peripheral Atypical Inflammatory Mediators Potentiate Neuroinflammation and Neurodegeneration
- PMID: 28790893
- PMCID: PMC5522882
- DOI: 10.3389/fncel.2017.00216
Brain and Peripheral Atypical Inflammatory Mediators Potentiate Neuroinflammation and Neurodegeneration
Abstract
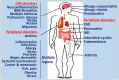
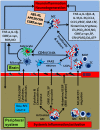
Neuroinflammatory response is primarily a protective mechanism in the brain. However, excessive and chronic inflammatory responses can lead to deleterious effects involving immune cells, brain cells and signaling molecules. Neuroinflammation induces and accelerates pathogenesis of Parkinson's disease (PD), Alzheimer's disease (AD) and Multiple sclerosis (MS). Neuroinflammatory pathways are indicated as novel therapeutic targets for these diseases. Mast cells are immune cells of hematopoietic origin that regulate inflammation and upon activation release many proinflammatory mediators in systemic and central nervous system (CNS) inflammatory conditions. In addition, inflammatory mediators released from activated glial cells induce neurodegeneration in the brain. Systemic inflammation-derived proinflammatory cytokines/chemokines and other factors cause a breach in the blood brain-barrier (BBB) thereby allowing for the entry of immune/inflammatory cells including mast cell progenitors, mast cells and proinflammatory cytokines and chemokines into the brain. These peripheral-derived factors and intrinsically generated cytokines/chemokines, α-synuclein, corticotropin-releasing hormone (CRH), substance P (SP), beta amyloid 1-42 (Aβ1-42) peptide and amyloid precursor proteins can activate glial cells, T-cells and mast cells in the brain can induce additional release of inflammatory and neurotoxic molecules contributing to chronic neuroinflammation and neuronal death. The glia maturation factor (GMF), a proinflammatory protein discovered in our laboratory released from glia, activates mast cells to release inflammatory cytokines and chemokines. Chronic increase in the proinflammatory mediators induces neurotoxic Aβ and plaque formation in AD brains and neurodegeneration in PD brains. Glial cells, mast cells and T-cells can reactivate each other in neuroinflammatory conditions in the brain and augment neuroinflammation. Further, inflammatory mediators from the brain can also enter into the peripheral system through defective BBB, recruit immune cells into the brain, and exacerbate neuroinflammation. We suggest that mast cell-associated inflammatory mediators from systemic inflammation and brain could augment neuroinflammation and neurodegeneration in the brain. This review article addresses the role of some atypical inflammatory mediators that are associated with mast cell inflammation and their activation of glial cells to induce neurodegeneration.
Keywords: brain; cytokines and chemokines; inflammatory mediators; mast cells; neurodegenerative diseases; neuroinflammation.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

