Differences between Dorsal and Ventral Striatum in the Sensitivity of Tonically Active Neurons to Rewarding Events
- PMID: 28790898
- PMCID: PMC5522860
- DOI: 10.3389/fnsys.2017.00052
Differences between Dorsal and Ventral Striatum in the Sensitivity of Tonically Active Neurons to Rewarding Events
Abstract
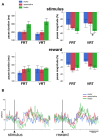
Within the striatum, cholinergic interneurons, electrophysiologically identified as tonically active neurons (TANs), represent a relatively homogeneous group in terms of their functional properties. They display typical pause in tonic firing in response to rewarding events which are of crucial importance for reinforcement learning. These responses are uniformly distributed throughout the dorsal striatum (i.e., motor and associative striatum), but it is unknown, at least in monkeys, whether differences in the modulation of TAN activity exist in the ventral striatum (i.e., limbic striatum), a region specialized for processing of motivational information. To address this issue, we examined the activity of dorsal and ventral TANs in two monkeys trained on a Pavlovian conditioning task in which a visual stimulus preceded the delivery of liquid reward by a fixed time interval. We found that the proportion of TANs responding to the stimulus predictive of reward did not vary significantly across regions (58%-80%), whereas the fraction of TANs responding to reward was higher in the limbic striatum (100%) compared to the motor (65%) and associative striatum (52%). By examining TAN modulation at the level of both the population and the individual neurons, we showed that the duration of pause responses to the stimulus and reward was longer in the ventral than in the dorsal striatal regions. Also, the magnitude of the pause was greater in ventral than dorsal striatum for the stimulus predictive of reward but not for the reward itself. We found similar region-specific differences in pause response duration to the stimulus when the timing of reward was less predictable (fixed replaced by variable time interval). Regional variations in the duration and magnitude of the pause response were transferred from the stimulus to reward when reward was delivered in the absence of any predictive stimulus. It therefore appears that ventral TANs exhibit stronger responses to rewarding stimuli, compared to dorsal TANs. The high proportion of responsive neurons, combined with particular response features, support the notion that the ventral TAN system can be driven by specific synaptic inputs arising from afferent sources distinct from those targeting the dorsal TAN system.
Keywords: basal ganglia; learning; motivation; prediction; primate.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources

