π-extended anthracenes as sensitive probes for mechanical stress
- PMID: 28791098
- PMCID: PMC5518546
- DOI: 10.1039/c5sc03297k
π-extended anthracenes as sensitive probes for mechanical stress
Abstract
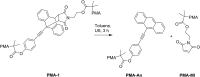
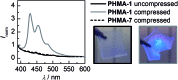
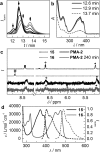
Smart molecular systems having the ability to report on mechanical strain or failure in polymers via alteration of their optical properties are of great interest in materials science. However, only limited attention has been devoted to targeted chromophore engineering to fine-tune their physicochemical properties. Here, we describe the synthesis of π-extended anthracenes that can be released from their respective maleimide Diels-Alder adducts through the application of mechanical stress in solution and in the solid state. We demonstrate the improvement of fluorescence quantum yield as well as the tuning of excitation and emission wavelengths while retaining their excellent mechanochemical properties laying the foundation for a new series of mechanophores whose spectral characteristics can be modularly adjusted.
Figures



References
-
- Li J., Nagamani C., Moore J. S. Acc. Chem. Res. 2015;48:2181–2190. - PubMed
-
- Groote R., Jakobs R. T. M., Sijbesma R. P. Polym. Chem. 2013;4:4846–4859.
-
- Brantley J. N., Wiggins K. M., Bielawski C. W. Polym. Int. 2013;62:2–12.
-
- Kean Z. S., Craig S. L. Polymer. 2012;53:1035–1048.
-
- Caruso M. M., Davis D. A., Shen Q., Odom S. A., Sottos N. R., White S. R., Moore J. S. Chem. Rev. 2009;109:5755–5798. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

