Peptide-based synthetic vaccines
- PMID: 28791117
- PMCID: PMC5529997
- DOI: 10.1039/c5sc03892h
Peptide-based synthetic vaccines
Abstract
Classically all vaccines were produced using live or attenuated microorganisms or parts of them. However, the use of whole organisms, their components or the biological process for vaccine production has several weaknesses. The presence of immunologically redundant biological components or biological impurities in such vaccines might cause major problems. All the disadvantageous of traditional vaccines might be overcome via the development of fully synthetic peptide-based vaccines. However, once minimal antigenic epitopes only are applied for immunisation, the immune responses are poor. The use of an adjuvant can overcome this obstacle; however, it may raise new glitches. Here we briefly summarise the current stand on peptide-based vaccines, discuss epitope and adjuvant design, and multi-epitope and nanoparticle-based vaccine approaches. This mini review discusses also the disadvantages and benefits associated with peptide-based vaccines. It proposes possible methods to overcome the weaknesses of the synthetic vaccine strategy and suggests future directions for its development.
Figures



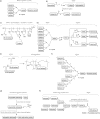


References
-
- Andersen P., Doherty T. M. Nat. Rev. Microbiol. 2005;3:656–662. - PubMed
-
- Wallach J. C., Ferrero M. C., Delpino M. V., Fossati C. A., Baldi P. C. Clin. Microbiol. Infect. 2008;14:805–807. - PubMed
-
- Steer A. C., Batzloff M. R., Mulholland K., Carapetis J. R. Curr. Opin. Infect. Dis. 2009;22:544–552. - PubMed
-
- Neefjes J., Jongsma M. L. M., Paul P., Bakke O. Nat. Rev. Immunol. 2011;11:823–836. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

