Generation of a Vero-Based Packaging Cell Line to Produce SV40 Gene Delivery Vectors for Use in Clinical Gene Therapy Studies
- PMID: 28791314
- PMCID: PMC5537168
- DOI: 10.1016/j.omtm.2017.06.007
Generation of a Vero-Based Packaging Cell Line to Produce SV40 Gene Delivery Vectors for Use in Clinical Gene Therapy Studies
Abstract
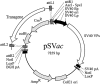
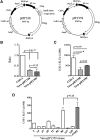
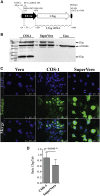
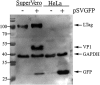
Replication-defective (RD) recombinant simian virus 40 (SV40)-based gene delivery vectors hold a great potential for clinical applications because of their presumed non-immunogenicity and capacity to induce immune tolerance to the transgene products in humans. However, the clinical use of SV40 vectors has been hampered by the lack of a packaging cell line that produces replication-competent (RC) free SV40 particles in the vector production process. To solve this problem, we have adapted the current SV40 vector genome used for the production of vector particles and generated a novel Vero-based packaging cell line named SuperVero that exclusively expresses the SV40 large T antigen. SuperVero cells produce similar numbers of SV40 vector particles compared to the currently used packaging cell lines, albeit in the absence of contaminating RC SV40 particles. Our unique SV40 vector platform named SVac paves the way to clinically test a whole new generation of SV40-based therapeutics for a broad range of important diseases.
Keywords: SV40 viral vectors; SVac; SuperVero; packaging cells.
Figures
Similar articles
-
A new packaging cell line for SV40 vectors that eliminates the generation of T-antigen-positive, replication-competent recombinants.Virology. 2002 Dec 20;304(2):155-9. doi: 10.1006/viro.2002.1791. Virology. 2002. PMID: 12504557
-
Packaging cells based on inducible gene amplification for the production of adeno-associated virus vectors.J Virol. 1998 Sep;72(9):7024-31. doi: 10.1128/JVI.72.9.7024-7031.1998. J Virol. 1998. PMID: 9696794 Free PMC article.
-
SV40-based gene therapy vectors: turning an adversary into a friend.Curr Opin Mol Ther. 2000 Oct;2(5):570-8. Curr Opin Mol Ther. 2000. PMID: 11249759 Review.
-
Potential of recombinant SV40-based vectors for gene therapy.Recent Pat DNA Gene Seq. 2007;1(2):93-9. doi: 10.2174/187221507780887045. Recent Pat DNA Gene Seq. 2007. PMID: 19075921 Review.
-
Transgene Expression and Host Cell Responses to Replication-Defective, Single-Cycle, and Replication-Competent Adenovirus Vectors.Genes (Basel). 2017 Feb 18;8(2):79. doi: 10.3390/genes8020079. Genes (Basel). 2017. PMID: 28218713 Free PMC article.
Cited by
-
Influence of gene modification in biological behaviors and responses of mouse lung telocytes to inflammation.J Transl Med. 2019 May 15;17(1):158. doi: 10.1186/s12967-019-1870-y. J Transl Med. 2019. PMID: 31092264 Free PMC article.
-
Transplantation for Primary Hyperoxaluria Type 1: Designing New Strategies in the Era of Promising Therapeutic Perspectives.Kidney Int Rep. 2020 Sep 24;5(12):2136-2145. doi: 10.1016/j.ekir.2020.09.022. eCollection 2020 Dec. Kidney Int Rep. 2020. PMID: 33305106 Free PMC article. Review.
-
The Evolution of Gene Therapy in the Treatment of Metabolic Liver Diseases.Genes (Basel). 2020 Aug 10;11(8):915. doi: 10.3390/genes11080915. Genes (Basel). 2020. PMID: 32785089 Free PMC article. Review.
-
Improving Treatment Options for Primary Hyperoxaluria.Drugs. 2022 Jul;82(10):1077-1094. doi: 10.1007/s40265-022-01735-x. Epub 2022 Jul 2. Drugs. 2022. PMID: 35779234 Free PMC article. Review.
-
How Simian Virus 40 Hijacks the Intracellular Protein Trafficking Pathway to Its Own Benefit … and Ours.Front Immunol. 2018 May 28;9:1160. doi: 10.3389/fimmu.2018.01160. eCollection 2018. Front Immunol. 2018. PMID: 29892296 Free PMC article. Review.
References
-
- Jiang H., Lillicrap D., Patarroyo-White S., Liu T., Qian X., Scallan C.D., Powell S., Keller T., McMurray M., Labelle A. Multiyear therapeutic benefit of AAV serotypes 2, 6, and 8 delivering factor VIII to hemophilia A mice and dogs. Blood. 2006;108:107–115. - PubMed
-
- Naldini L., Blömer U., Gallay P., Ory D., Mulligan R., Gage F.H., Verma I.M., Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. - PubMed
-
- Perrin G.Q., Zolotukhin I., Sherman A., Biswas M., de Jong Y.P., Terhorst C., Davidoff A.M., Herzog R.W. Dynamics of antigen presentation to transgene product-specific CD4(+) T cells and of Treg induction upon hepatic AAV gene transfer. Mol. Ther. Methods Clin. Dev. 2016;3:16083–16088. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

