Juglone reduces growth and migration of U251 glioblastoma cells and disrupts angiogenesis
- PMID: 28791366
- PMCID: PMC5652942
- DOI: 10.3892/or.2017.5878
Juglone reduces growth and migration of U251 glioblastoma cells and disrupts angiogenesis
Abstract
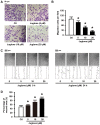
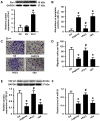
Accumulating data show that prolylisomerase (Pin1) is overexpressed in human glioblastoma multiforme (GBM) specimens. Therefore, Pin1 inhibitors should be investigated as a new chemotherapeutic drug that may enhance the clinical management of human gliomas. Recently, juglone, a Pin1 inhibitor, was shown to exhibit potent anticancer activity in various tumor cells, but its role in human glioma cells remains unknown. In the present study, we determined if juglone exerts antitumor effects in the U251 human glioma cell line and investigated its potential underlying molecular mechanisms. Cell survival, apoptosis, migration, angiogenesis and molecular targets were identified with multiple detection techniques including the MTT cell proliferation assay, dual acridine orange/ethidium bromide staining, electron microscopy, transwell migration assay, chick chorioallantoic membrane assay, quantitative real-time polymerase chain reaction and immunoblotting. The results showed that 5-20 µM juglone markedly suppressed cell proliferation, induced apoptosis, and enhanced caspase-3 activity in U251 cells in a dose- and time-dependent manner. Moreover, juglone inhibited cell migration and the formation of new blood vessels. At the molecular level, juglone markedly suppressed Pin1 levels in a time-dependent manner. TGF-β1/Smad signaling, a critical upstream regulator of miR-21, was also suppressed by juglone. Moreover, the transient overexpression of Pin1 reversed its antitumor effects in U251 cells and inhibited juglone-mediated changes to the TGF-β1/miR-21 signaling pathway. These findings suggest that juglone inhibits cell growth by causing apoptosis, thereby inhibiting the migration of U251 glioma cells and disrupting angiogenesis; and that Pin1 is a critical target for juglone's antitumor activity. The present study provides evidence that juglone has in vitro efficacy against glioma. Therefore, additional studies are warranted to examine the clinical potential of juglone in human gliomas.
Figures



Similar articles
-
Ursolic acid inhibits proliferation and induces apoptosis in human glioblastoma cell lines U251 by suppressing TGF-β1/miR-21/PDCD4 pathway.Basic Clin Pharmacol Toxicol. 2012 Aug;111(2):106-12. doi: 10.1111/j.1742-7843.2012.00870.x. Epub 2012 Mar 21. Basic Clin Pharmacol Toxicol. 2012. PMID: 22353043
-
Molecular biological mechanism of action in cancer therapies: Juglone and its derivatives, the future of development.Biomed Pharmacother. 2022 Apr;148:112785. doi: 10.1016/j.biopha.2022.112785. Epub 2022 Mar 7. Biomed Pharmacother. 2022. PMID: 35272138
-
Overexpression of PIN1 Enhances Cancer Growth and Aggressiveness with Cyclin D1 Induction in EBV-Associated Nasopharyngeal Carcinoma.PLoS One. 2016 Jun 3;11(6):e0156833. doi: 10.1371/journal.pone.0156833. eCollection 2016. PLoS One. 2016. PMID: 27258148 Free PMC article.
-
Juglone suppresses vasculogenic mimicry in glioma through inhibition of HuR-mediated VEGF-A expression.Biochem Pharmacol. 2024 Sep;227:116458. doi: 10.1016/j.bcp.2024.116458. Epub 2024 Aug 3. Biochem Pharmacol. 2024. PMID: 39102993
-
Tumor Development and Angiogenesis in Adult Brain Tumor: Glioblastoma.Mol Neurobiol. 2020 May;57(5):2461-2478. doi: 10.1007/s12035-020-01892-8. Epub 2020 Mar 9. Mol Neurobiol. 2020. PMID: 32152825 Free PMC article. Review.
Cited by
-
PIN1 in Cell Cycle Control and Cancer.Front Pharmacol. 2018 Nov 26;9:1367. doi: 10.3389/fphar.2018.01367. eCollection 2018. Front Pharmacol. 2018. PMID: 30534074 Free PMC article. Review.
-
Juglone Encapsulation in PLGA Nanoparticles Improves Solubility and Enhances Apoptosis in HeLa Cells.Cell Biochem Biophys. 2025 Feb 14. doi: 10.1007/s12013-025-01691-9. Online ahead of print. Cell Biochem Biophys. 2025. PMID: 39948289
-
Preparation and Antiproliferative Activity Evaluation of Juglone-Loaded BSA Nanoparticles.Adv Pharm Bull. 2022 Aug;12(4):818-827. doi: 10.34172/apb.2022.087. Epub 2022 Jan 3. Adv Pharm Bull. 2022. PMID: 36415643 Free PMC article.
-
Function of PIN1 in Cancer Development and Its Inhibitors as Cancer Therapeutics.Front Cell Dev Biol. 2020 Mar 17;8:120. doi: 10.3389/fcell.2020.00120. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32258027 Free PMC article. Review.
-
Pharmacotherapeutic potential of walnut (Juglans spp.) in age-related neurological disorders.IBRO Neurosci Rep. 2022 Nov 26;14:1-20. doi: 10.1016/j.ibneur.2022.10.015. eCollection 2023 Jun. IBRO Neurosci Rep. 2022. PMID: 36507190 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

